Korean J Physiol Pharmacol.
2018 Mar;22(2):155-162. 10.4196/kjpp.2018.22.2.155.
The antidepressant action of 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid is mediated by phosphorylation of histone deacetylase 5
- Affiliations
-
- 1Department of Biomedical Sciences, Graduate School of Biomedical Science and Engineering, Seoul 04763, Korea. hyeonson@hanyang.ac.kr, yongsk@hanyang.ac.kr
- 2Department of Biochemistry and Molecular Biology, College of Medicine, Hanyang University, Seoul 04763, Korea.
- KMID: 2410105
- DOI: http://doi.org/10.4196/kjpp.2018.22.2.155
Abstract
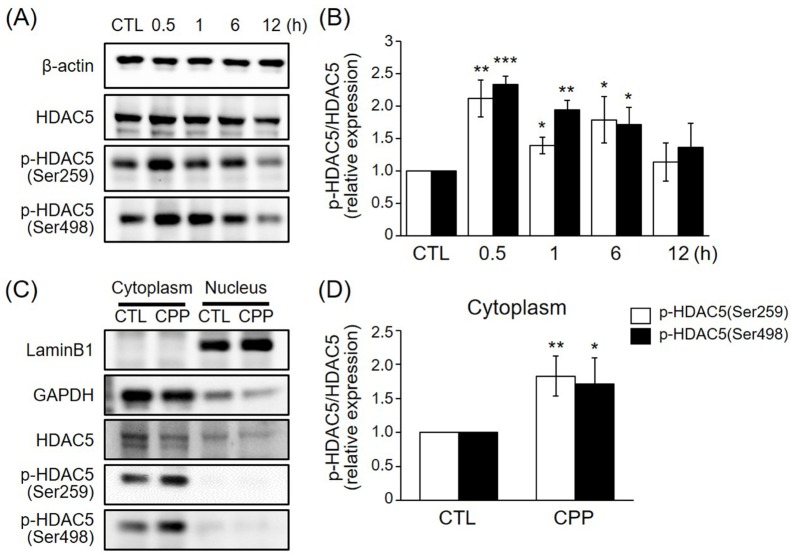
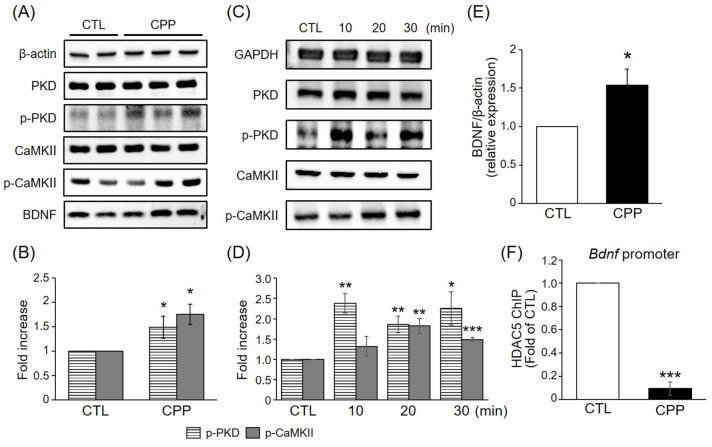
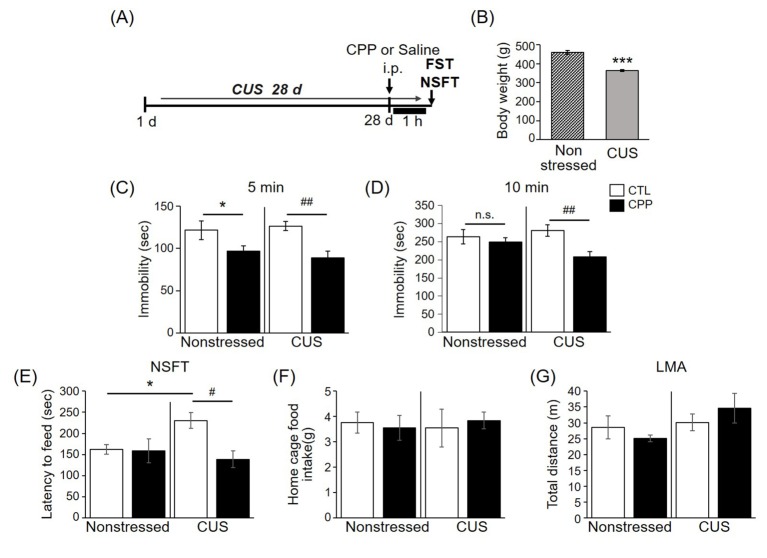
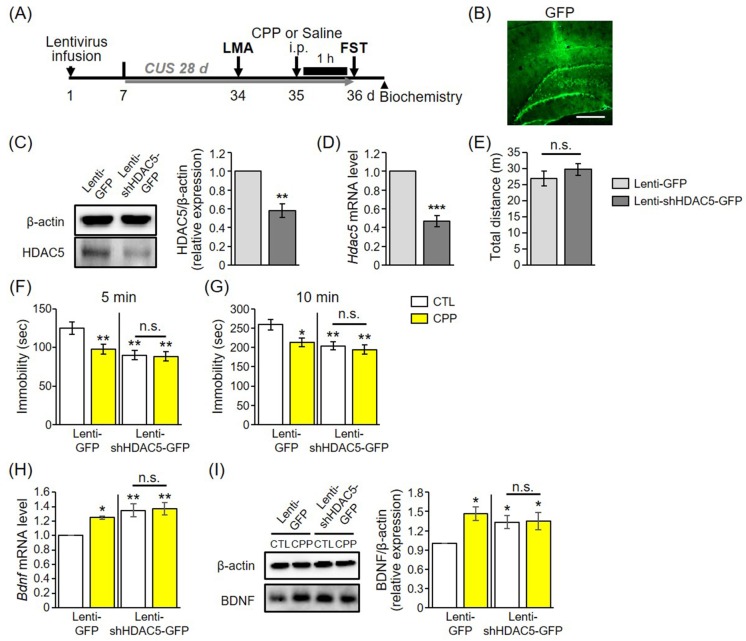
- 3-(2-Carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP), a competitive N-methyl-D-aspartate (NMDA) receptor antagonist, produces rapid antidepressant-like effects in animal models of depression. However, the molecular mechanisms underlying these behavioral actions remain unknown. Here, we demonstrate that CPP rapidly stimulates histone deacetylase (HDAC) 5 phosphorylation and nuclear export in rat hippocampal neurons. These effects are accompanied by calcium/calmodulin kinase II (CaMKII) and protein kinase D (PKD) phosphorylation. Behavioral experiments revealed that viral-mediated hippocampal knockdown of HDAC5 blocked the antidepressant effects of CPP in stressed animals. Taken together, our results imply that CPP acts via HDAC5 and suggest that HDAC5 is a common regulator contributing to the antidepressant actions of NMDA receptor antagonists such as CPP.
MeSH Terms
Figure
Reference
-
1. Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012; 338:68–72. PMID: 23042884.
Article2. Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009; 33:699–771. PMID: 19428491.
Article3. Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Lepack A, Majik MS, Jeong LS, Banasr M, Son H, Duman RS. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012; 18:1413–1417. PMID: 22885997.
Article4. MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry. 2008; 64:880–883. PMID: 18722590.
Article5. Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002; 22:6810–6818. PMID: 12151561.
Article6. Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011; 69:754–761. PMID: 21292242.
Article7. Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006; 9:519–525. PMID: 16501568.
Article8. Sun H, Kennedy PJ, Nestler EJ. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology. 2013; 38:124–137. PMID: 22692567.
Article9. Belfield JL, Whittaker C, Cader MZ, Chawla S. Differential effects of Ca2+ and cAMP on transcription mediated by MEF2D and cAMP-response element-binding protein in hippocampal neurons. J Biol Chem. 2006; 281:27724–27732. PMID: 16870618.10. Flavell SW, Kim TK, Gray JM, Harmin DA, Hemberg M, Hong EJ, Markenscoff-Papadimitriou E, Bear DM, Greenberg ME. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron. 2008; 60:1022–1038. PMID: 19109909.
Article11. Choi M, Lee SH, Wang SE, Ko SY, Song M, Choi JS, Kim YS, Duman RS, Son H. Ketamine produces antidepressant-like effects through phosphorylation-dependent nuclear export of histone deacetylase 5 (HDAC5) in rats. Proc Natl Acad Sci U S A. 2015; 112:15755–15760. PMID: 26647181.
Article12. Lehmann J, Schneider J, McPherson S, Murphy DE, Bernard P, Tsai C, Bennett DA, Pastor G, Steel DJ, Boehm C, et al. CPP, a selective N-methyl-D-aspartate (NMDA)-type receptor antagonist: characterization in vitro and in vivo. J Pharmacol Exp Ther. 1987; 240:737–746. PMID: 2882014.13. Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011; 475:91–95. PMID: 21677641.
Article14. Martin KP, Wellman CL. NMDA receptor blockade alters stressinduced dendritic remodeling in medial prefrontal cortex. Cereb Cortex. 2011; 21:2366–2373. PMID: 21383235.
Article15. Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010; 107:2669–2674. PMID: 20133768.16. McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci U S A. 2000; 97:14400–14405. PMID: 11114197.
Article17. Linseman DA, Bartley CM, Le SS, Laessig TA, Bouchard RJ, Meintzer MK, Li M, Heidenreich KA. Inactivation of the myocyte enhancer factor-2 repressor histone deacetylase-5 by endogenous Ca2+/calmodulin-dependent kinase II promotes depolarization-mediated cerebellar granule neuron survival. J Biol Chem. 2003; 278:41472–41481. PMID: 12896970.18. Chawla S, Vanhoutte P, Arnold FJ, Huang CL, Bading H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J Neurochem. 2003; 85:151–159. PMID: 12641737.
Article19. Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, McKinsey TA, Olson EN, Jin ZG. Protein kinase D-dependent phosphorylation and nuclear export of histone deacetylase 5 mediates vascular endothelial growth factor-induced gene expression and angiogenesis. J Biol Chem. 2008; 283:14590–14599. PMID: 18332134.
Article20. Delehanty LL, Bullock GC, Goldfarb AN. Protein kinase D-HDAC5 signaling regulates erythropoiesis and contributes to erythropoietin cross-talk with GATA1. Blood. 2012; 120:4219–4228. PMID: 22983445.
Article21. Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008; 455:894–902. PMID: 18923511.
Article22. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006; 59:1116–1127. PMID: 16631126.
Article23. Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008; 33:320–331. PMID: 17406647.
Article24. Malykhin NV, Coupland NJ. Hippocampal neuroplasticity in major depressive disorder. Neuroscience. 2015; 309:200–213. PMID: 25934030.
Article25. Jagirdar R, Drexel M, Bukovac A, Tasan RO, Sperk G. Expression of class II HDACs in two mouse models of temporal lobe epilepsy. J Neurochem. 2015; 136:717–730. PMID: 26603269.26. Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008; 33:18–41. PMID: 17728696.
Article27. Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011; 34:89–103. PMID: 21438687.
Article28. Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016; 533:481–486. PMID: 27144355.
Article29. Tan S, Lam WP, Wai MS, Yu WH, Yew DT. Chronic ketamine administration modulates midbrain dopamine system in mice. PLoS One. 2012; 7:e43947. PMID: 22937133.
Article30. Hustveit O, Maurset A, Oye I. Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol Toxicol. 1995; 77:355–359. PMID: 8835358.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activation of ATM-dependent DNA Damage Signal Pathway by a Histone Deacetylase Inhibitor, Trichostatin A
- The Role of Histone Acetylation in Mesenchymal Stem Cell Differentiation
- A Case of Allergic Contact Cheilitis from Propyl Gallate
- Effects of Histone Deacetylase Inhibitor (Valproic Acid) on the Expression of Hypoxia-inducible Factor-1 Alpha in Human Retinal Müller Cells
- New Promising Therapeutic Modalities for Thyroid Cancers: Histone Deacetylase Inhibitor, PPAR-gamma Agonist, and Retinoic Acid