Korean J Ophthalmol.
2017 Feb;31(1):80-85. 10.3341/kjo.2017.31.1.80.
Effects of Histone Deacetylase Inhibitor (Valproic Acid) on the Expression of Hypoxia-inducible Factor-1 Alpha in Human Retinal Müller Cells
- Affiliations
-
- 1Department of Ophthalmology and Inha Vision Science Laboratory, Inha University School of Medicine, Incheon, Korea. hschin@inha.ac.kr
- KMID: 2368682
- DOI: http://doi.org/10.3341/kjo.2017.31.1.80
Abstract
- PURPOSE
To evaluate the effects of valproic acid (VPA), a histone deacetylase inhibitor (HDACI), on the expression of hypoxia-inducible factor-1 alpha (HIF-1α) and vascular endothelial growth factor (VEGF) in human retinal Müller cells under hypoxic conditions.
METHODS
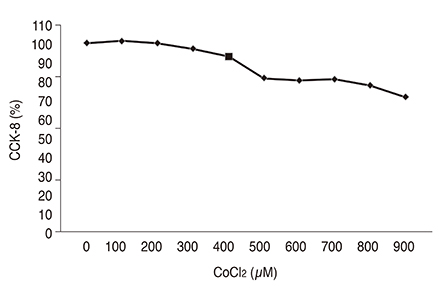
Chemical hypoxia was induced in human retinal Müller cells (MIO-M1) by treatment with increasing concentrations of cobalt(II) chloride (CoCl₂). Müller cells were also treated with a set concentration of CoCl₂, along with various concentrations of VPA. The expression of HIF-1α and VEGF in the treated Müller cells was determined by enzyme-linked immunosorbent assay.
RESULTS
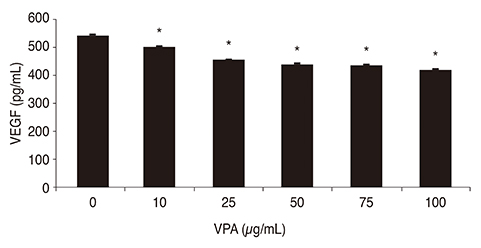
Exposure of human retinal Müller cells to increasing concentrations of CoCl₂ produced a dose-dependent increase in HIF-1α expression. The addition of increasing concentrations of VPA lead to a dose-dependent decrease in expression of HIF-1α and VEGF in Müller cells exposed to a set concentration of CoCl₂.
CONCLUSIONS
HDACI VPA downregulated the expressions of HIF-1α and VEGF in human retinal Müller cells under hypoxic conditions. Using HDACI to target HIF-1α expression in Müller cells could be a new therapeutic strategy for the treatment of retinal vascular diseases.
Keyword
MeSH Terms
-
Anoxia
Enzyme-Linked Immunosorbent Assay
Ependymoglial Cells
Histone Deacetylase Inhibitors*
Histone Deacetylases*
Histones*
Humans*
Retinaldehyde*
Valproic Acid
Vascular Diseases
Vascular Endothelial Growth Factor A
Histone Deacetylase Inhibitors
Histone Deacetylases
Histones
Retinaldehyde
Valproic Acid
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Congdon N, O'Colmain B, Klaver CC, et al. Causes a nd prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004; 122:477–485.2. Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003; 290:2057–2060.3. Stalmans I, Ng YS, Rohan R, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002; 109:327–336.4. Haigh JJ, Morelli PI, Gerhardt H, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003; 262:225–241.5. Marneros AG, Fan J, Yokoyama Y, et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005; 167:1451–1459.6. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–1487.7. Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994; 118:445–450.8. Pierce EA, Avery RL, Foley ED, et al. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995; 92:905–909.9. Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev. 1997; 13:37–50.10. Aiello LP, Northrup JM, Keyt BA, et al. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995; 113:1538–1544.11. Stone J, Itin A, Alon T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995; 15(7 Pt 1):4738–4747.12. Stone J, Chan-Ling T, Pe'er J, et al. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1996; 37:290–299.13. Bai Y, Ma JX, Guo J, et al. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009; 219:446–454.14. Wang J, Xu X, Elliott MH, et al. Muller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010; 59:2297–2305.15. Kaelin WG Jr. The von Hippel-Lindau tumor suppressor protein and clear cell renal carcinoma. Clin Cancer Res. 2007; 13:680s–684s.16. Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007; 26:281–290.17. Ke Q, Costa M. Hypoxia-inducible f actor-1 (HIF-1). Mol Pharmacol. 2006; 70:1469–1480.18. Pili R, Donehower RC. Is HIF-1 alpha a valid therapeutic target? J Natl Cancer Inst. 2003; 95:498–499.19. Welsh SJ, Powis G. Hypoxia inducible factor as a cancer drug target. Curr Cancer Drug Targets. 2003; 3:391–405.20. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003; 3:721–732.21. Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003; 2:803–811.22. Powis G, Kirkpatrick L. Hypoxia inducible factor-1alpha as a cancer drug target. Mol Cancer Ther. 2004; 3:647–654.23. Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004; 4:437–447.24. Poon E, Harris AL, Ashcroft M. Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev Mol Med. 2009; 11:e26.25. Gottlicher M, Minucci S, Zhu P, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001; 20:6969–6978.26. Zhang ZH, Hao CL, Liu P, et al. Valproic acid inhibits tumor angiogenesis in mice transplanted with Kasumi1 leukemia cells. Mol Med Rep. 2014; 9:443–449.27. Shan Z, Feng-Nian R, Jie G, Ting Z. Effects of valproic acid on proliferation, apoptosis, angiogenesis and metastasis of ovarian cancer in vitro and in vivo. Asian Pac J Cancer Prev. 2012; 13:3977–3982.28. Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol. 2001; 13:477–483.29. Drummond DC, Noble CO, Kirpotin DB, et al. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol. 2005; 45:495–528.30. Johnstone RW, Licht JD. Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell. 2003; 4:13–18.31. DeNiro M, Al-Halafi A, Al-Mohanna FH, et al. Pleiotropic effects of YC-1 selectively inhibit pathological retinal neovascularization and promote physiological revascularization in a mouse model of oxygen-induced retinopathy. Mol Pharmacol. 2010; 77:348–367.32. Adams JM, Difazio LT, Rolandelli RH, et al. HIF-1: a key mediator in hypoxia. Acta Physiol Hung. 2009; 96:19–28.33. Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995; 92:10457–10461.34. Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010; 86:236–242.35. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.36. Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A. 2001; 98:9630–9635.37. Mie Lee Y, Kim SH, Kim HS, et al. Inhibition of hypoxia-induced angiogenesis by FK228, a specific histone deacetylase inhibitor, via suppression of HIF-1alpha activity. Biochem Biophys Res Commun. 2003; 300:241–246.38. Williams RJ. Trichostatin A, an inhibitor of histone deacetylase, inhibits hypoxia-induced angiogenesis. Expert Opin Investig Drugs. 2001; 10:1571–1573.39. Biermann J, Grieshaber P, Goebel U, et al. Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010; 51:526–534.40. Zhang Z, Qin X, Zhao X, et al. Valproic acid regulates antioxidant enzymes and prevents ischemia/reperfusion injury in the rat retina. Curr Eye Res. 2012; 37:429–437.41. Zhang Z, Qin X, Tong N, et al. Valproic acid-mediated neuroprotection in retinal ischemia injury via histone deacetylase inhibition and transcriptional activation. Exp Eye Res. 2012; 94:98–108.42. Jeong JW, Bae MK, Ahn MY, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002; 111:709–720.43. Kim MS, Kwon HJ, Lee YM, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001; 7:437–443.44. Kong X, Lin Z, Liang D, et al. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha. Mol Cell Biol. 2006; 26:2019–2028.45. Qian DZ, Kachhap SK, Collis SJ, et al. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res. 2006; 66:8814–8821.46. Lin M, Chen Y, Jin J, et al. Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Muller cells. Diabetologia. 2011; 54:1554–1566.47. Mowat FM, Luhmann UF, Smith AJ, et al. HIF-1alpha and HIF-2alpha are differentially activated in distinct cell populations in retinal ischaemia. PLoS One. 2010; 5:e11103.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Valproic acid, a Histone Deacetylase Inhibitor, on the Expression of Pluripotency and Neural Crest Specific Marker Genes in Murine Multipotent Skin Precursor Cells
- ACY-241, a histone deacetylase 6 inhibitor, suppresses the epithelial–mesenchymal transition in lung cancer cells by downregulating hypoxia-inducible factor-1 alpha
- Epigenetically Upregulated T-Type Calcium Channels Contribute to Abnormal Proliferation of Embryonic Neural Progenitor Cells Exposed to Valproic Acid
- HDAC Inhibition by Valproic Acid Induces Neuroprotection and Improvement of PD-like Behaviors in LRRK2 R1441G Transgenic Mice
- In Vitro and In Vivo Radiosensitizing Effect of Valproic Acid on Fractionated Irradiation