Ann Lab Med.
2018 Jul;38(4):355-361. 10.3343/alm.2018.38.4.355.
Comparison of Four Automated Carcinoembryonic Antigen Immunoassays: ADVIA Centaur XP, ARCHITECT I2000sr, Elecsys E170, and Unicel Dxi800
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. ejoh@catholic.ac.kr
- 2Catholic Laboratory Development and Evaluation Center, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Laboratory Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
- 4Department of Laboratory Medicine, Samkwang Medical Laboratories, Seoul, Korea.
- KMID: 2408075
- DOI: http://doi.org/10.3343/alm.2018.38.4.355
Abstract
- BACKGROUND
Carcinoembryonic antigen (CEA) is one of the tumor markers available for evaluating disease progression status after initial therapy and monitoring subsequent treatment modalities in colorectal, gastrointestinal, lung, and breast carcinoma. We evaluated the correlations and differences between widely used, automated CEA immunoassays at four different medical laboratories.
METHODS
In total, 393 serum samples with CEA ranging from 3.0 to 1,000 ng/mL were analyzed on ADVIA Centaur XP (Siemens Diagnostics, Tarrytown, NY, USA), ARCHITECT i2000sr (Abbott Diagnostics, Abbott Park, IL, USA), Elecsys E170 (Roche Diagnostics, Indianapolis, IN, USA), and Unicel DxI800 (Beckman Coulter, Fullerton, CA, USA), and the results were compared. Deming regression, Passing-Bablok regression, and Bland-Altman analyses were performed to evaluate the data correlation and % differences among these assays.
RESULTS
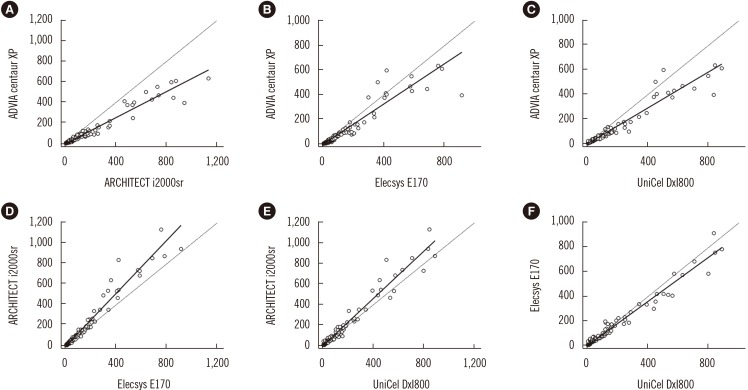
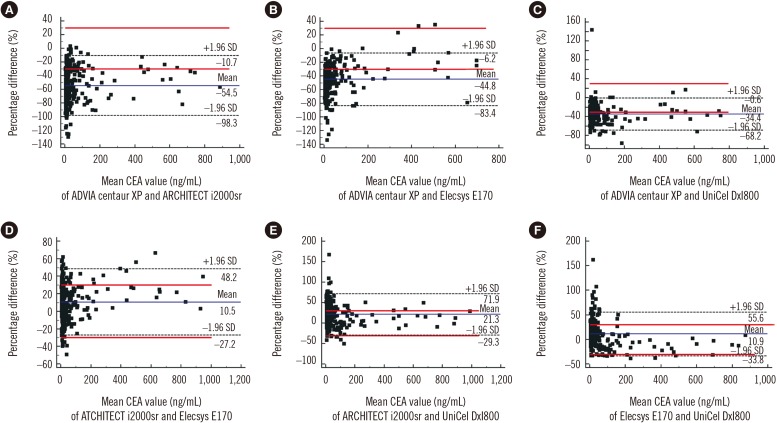
Deming regression analysis of data from Elecsys E170 and UniCel DxI800 showed good correlation (y=3.1615+0.8970x). According to Bland-Altman plot, no statistically significant bias (−1.78 ng/mL [95% confidence interval: −4.02 to 0.46]) was observed between Elecsys E170 and UniCel DxI800. However, the relative differences of CEA concentrations between assays exceeded the acceptable limit of 30%. Regarding the agreement of positivity with cut-off value 5.0 ng/mL, ARCHITECT i2000sr and Elecsys E170 showed the highest agreement (95.2%), whereas ADVIA Centaur XP and ARCHITECT i2000sr showed the lowest agreement (70.7%).
CONCLUSIONS
Agreements between automated CEA immunoassays are variable, and individual CEA concentrations may differ significantly between assays. Standardization of serum CEA concentrations and further harmonization are needed.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
False-positive Elevations in Carcinoembryonic Antigen Levels at a Health Screening Center
Soie Chung
Lab Med Online. 2019;9(3):146-152. doi: 10.3343/lmo.2019.9.3.146.
Reference
-
1. Jeon BG, Shin R, Chung JK, Jung IM, Heo SC. Individualized cutoff value of the preoperative carcinoembryonic antigen level is necessary for optimal use as a prognostic marker. Ann Coloproctol. 2013; 29:106–114. PMID: 23862128.2. Peng Y, Wang L, Gu J. Elevated preoperative carcinoembryonic antigen (CEA) and Ki67 is predictor of decreased survival in IIA stage colon cancer. World J Surg. 2013; 37:208–213. PMID: 23052808.3. Lawicki S, Glazewska EK, Sobolewska M, Bedkowska GE, Szmitkowski M. Plasma levels and diagnostic utility of macrophage colony-stimulating factor, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinases-1 as new biomarkers of breast cancer. Ann Lab Med. 2016; 36:223–229. PMID: 26915610.4. Kim CG, Ahn JB, Jung M, Beom SH, Heo SJ, Kim JH, et al. Preoperative serum carcinoembryonic antigen level as a prognostic factor for recurrence and survival after curative resection followed by adjuvant chemotherapy in stage III colon cancer. Ann Surg Oncol. 2017; 24:227–235. PMID: 27699609.5. Maeda R, Suda T, Hachimaru A, Tochii D, Tochii S, Takagi Y. Clinical significance of preoperative carcinoembryonic antigen level in patients with clinical stage IA non-small cell lung cancer. J Thorac Dis. 2017; 9:176–186. PMID: 28203421.6. Gold P. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965; 121:439–462. PMID: 14270243.7. Saito G, Sadahiro S, Kamata H, Miyakita H, Okada K, Tanaka A, et al. Monitoring of serum carcinoembryonic antigen levels after curative resection of colon cancer: cutoff values determined according to preoperative levels enhance the diagnostic accuracy for recurrence. Oncology. 2017; 92:276–282. PMID: 28178692.8. Choi SI, Jang MA, Jeon BR, Shin HB, Lee YK, Lee YW. Clinical usefulness of human epididymis protein 4 in lung cancer. Ann Lab Med. 2017; 37:526–530. PMID: 28840992.9. Cho YY, Chun S, Lee SY, Chung JH, Park HD, Kim SW. Performance evaluation of the serum thyroglobulin assays with immunochemiluminometric assay and immunoradiometric assay for differentiated thyroid cancer. Ann Lab Med. 2016; 36:413–419. PMID: 27374705.10. Karen LC, Viswanath D. Immunoassay methods. In : Sittampalam GS, Coussens NP, editors. Assay guidance manual [Internet]. Bethesda, MD: Eli Lilly & Company and the National Center for Advancing Translational Sciences;2014. p. 223–266.11. Miller WG, Tate JR, Barth JH, Jones GR. Harmonization: the sample, the measurement, and the report. Ann Lab Med. 2014; 34:187–197. PMID: 24790905.12. Matsushita H, Xu J, Kuroki M, Kondo A, Inoue E, Teramura Y, et al. Establishment and evaluation of a new chemiluminescent enzyme immunoassay for carcinoembryonic antigen adapted to the fully automated ACCESS system. Eur J Clin Chem Clin Biochem. 1996; 34:829–835. PMID: 8933107.13. Akbas N, Schryver PG, Algeciras-Schimnich A, Baumann NA, Block DR, Budd JR, et al. Evaluation of Beckman Coulter DxI 800 immunoassay system using clinically oriented performance goals. Clin Biochem. 2014; 47:158–163.14. Zhang GM, Guo XX, Ma XB, Zhang GM. Reference intervals of alphafetoprotein and carcinoembryonic antigen in the apparently healthy population. Med Sci Monit. 2016; 22:4875–4880. PMID: 27941709.15. Zhang K, Huo H, Lin G, Yue Y, Wang Q, Li J. A long way to go for the harmonization of four immunoassays for carcinoembryonic antigen. Clin Chim Acta. 2016; 454:15–19. PMID: 26721316.16. Sturgeon C. Standardization of tumor markers-priorities identified through external quality assessment. Scand J Clin Lab Invest Suppl. 2016; 245:S94–S99. PMID: 27542005.17. Twomey PJ. How to use difference plots in quantitative method comparison studies. Ann Clin Biochem. 2006; 43:124–129. PMID: 16536914.18. Park Y, Park Y, Park J, Kim HS. Evaluation of the UniCel DxI 800 immunoassay analyzer in measuring five tumor markers. Yonsei Med J. 2012; 53:557–564. PMID: 22477000.19. Falzarano R, Viggiani V, Michienzi S, Longo F, Tudini S, Frati L, et al. Evaluation of a CLEIA automated assay system for the detection of a panel of tumor markers. Tumour Biol. 2013; 34:3093–3100. PMID: 23775009.20. Bilic-Zulle L. Comparison of methods: Passing and Bablok regression. Biochem Med (Zagreb). 2011; 21:49–52. PMID: 22141206.21. Bowen RA, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochem Med (Zagreb). 2014; 24:31–44. PMID: 24627713.22. Laurence DJ, Turberville C, Anderson SG, Neville AM. First British standard for carcinoembryonic antigen (CEA). Br J Cancer. 1975; 32:295–299. PMID: 822862.23. Sorensen CG, Karlsson WK, Pommergaard HC, Burcharth J, Rosenberg J. The diagnostic accuracy of carcinoembryonic antigen to detect colorectal cancer recurrence-A systematic review. Int J Surg. 2016; 25:134–144. PMID: 26700203.24. Bjerner J, Hogetveit A, Wold Akselberg K, Vangsnes K, Paus E, Bjoro T, et al. Reference intervals for carcinoembryonic antigen (CEA), CA125, MUC1, Alfa-foeto-protein (AFP), neuron-specific enolase (NSE) and CA19.9 from the NORIP study. Scand J Clin Lab Invest. 2008; 68:703–713. PMID: 18609108.25. Qin X, Lin L, Mo Z, Lv H, Gao Y, Tan A, et al. Reference intervals for serum alpha-fetoprotein and carcinoembryonic antigen in Chinese Han ethnic males from the Fangchenggang Area Male Health and Examination Survey. Int J Biol Markers. 2011; 26:65–71. PMID: 21337313.26. Lao X, Yang D, Mo Z, Gao Y, Deng Y, Qin X, et al. Reference intervals for alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) in Guangxi Zhuang ethnic males from the FAMHES Project. Clin Lab. 2016; 62:955–961. PMID: 27349024.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Two Automated Immunoassays for the Detection of Anti-Hepatitis A Virus Total Immunoglobulin and IgM
- Comparison of 3 Automated Immunoassays for Detection of Anti-Hepatitis A Virus Immunoglobulin M in a Tertiary Care Hospital
- Comparison of Three Assay Systems for Qualitative and Quantitative Results of Hepatitis B Surface Antibody
- Comparison Between ADVIA Centaur XP and cobas e801 for Prostate-Specific Antigen Assays using Large-Scale Clinical Samples
- Comparison of 3 Automated Immunoassays for Hepatitis B Surface Antigen