J Breast Cancer.
2015 Sep;18(3):218-224. 10.4048/jbc.2015.18.3.218.
Lentivirus-Mediated Short-Hairpin RNA Targeting Protein Phosphatase 4 Regulatory Subunit 1 Inhibits Growth in Breast Cancer
- Affiliations
-
- 1Department of Laboratory, The Affiliated Ningde Municipal Hospital of Fujian Medical University, Ningde, China. yuying_qi@126.com
- 2Department of Oncological Surgery, The Affiliated Ningde Municipal Hospital of Fujian Medical University, Ningde, China.
- KMID: 2407568
- DOI: http://doi.org/10.4048/jbc.2015.18.3.218
Abstract
- PURPOSE
Protein phosphatase 4 regulatory subunit 1 (PP4R1), as an interaction partner of the catalytic serine/threonine-protein phosphatase 4 catalytic subunit has been shown to involve in cellular processes and nuclear factor kappaB signaling. However, the functions of PP4R1 in human breast cancers remain unclear. This study is designed to explore the effect of PP4R1 knockdown on the biological characteristics of breast cancer cells.
METHODS
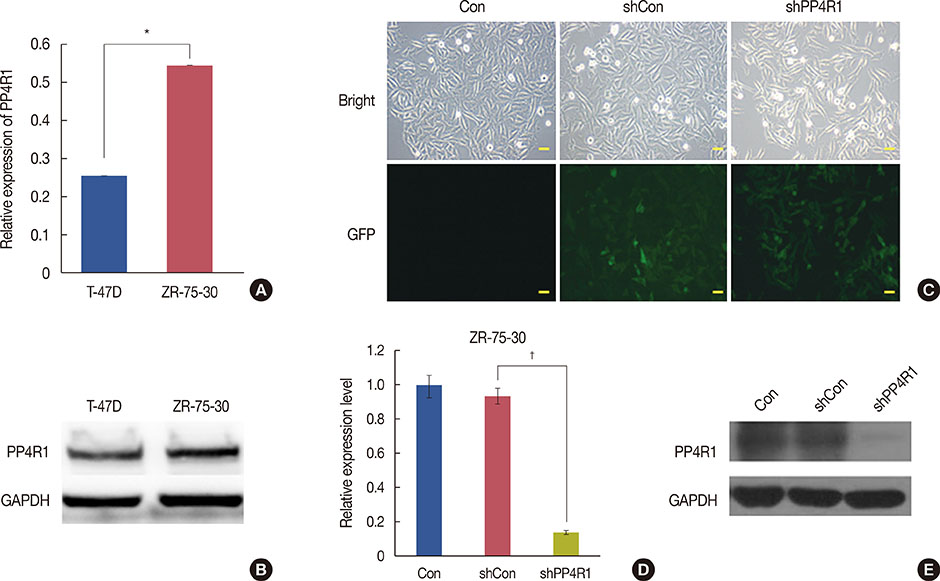
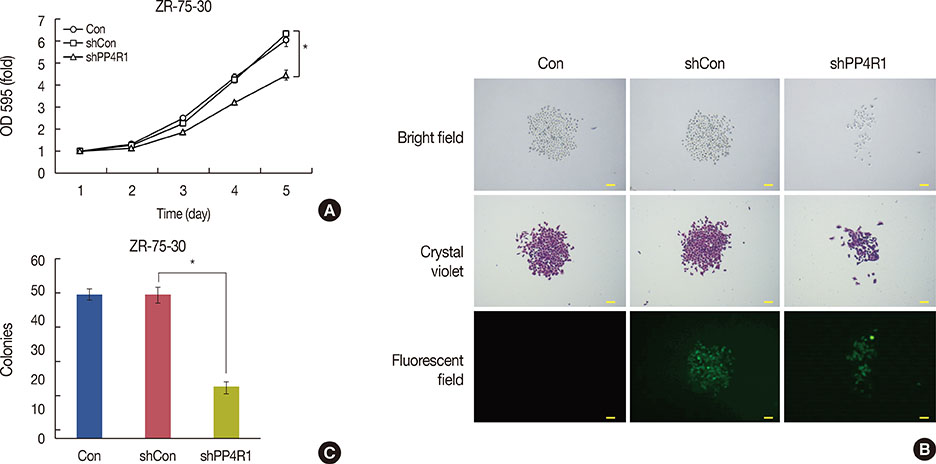
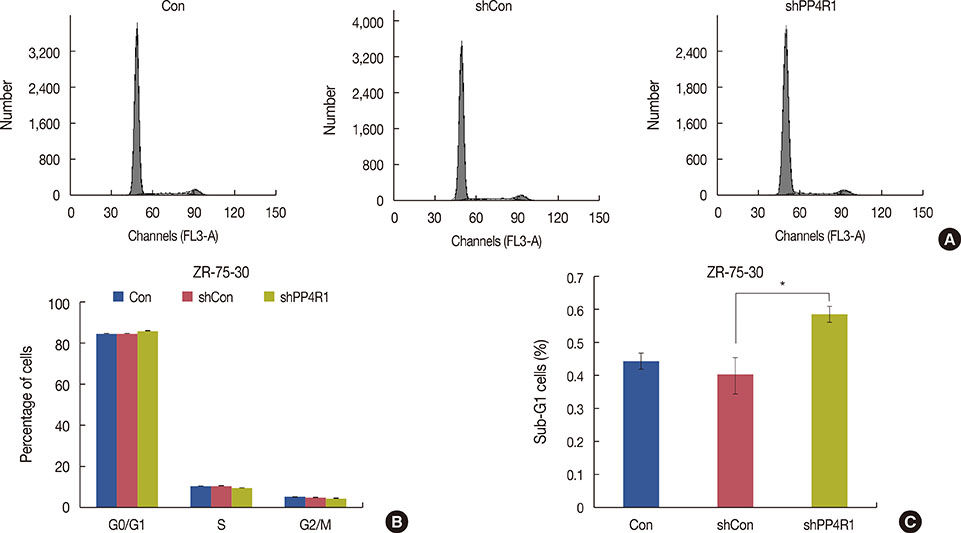
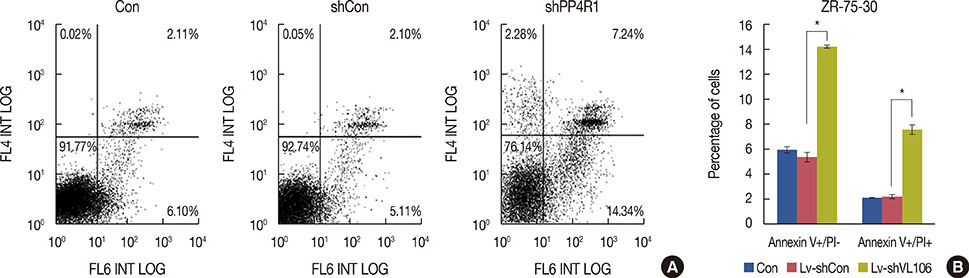
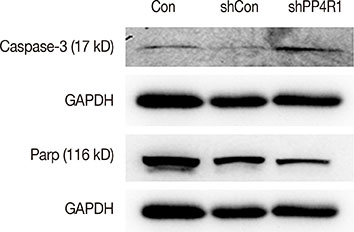
A lentivirus-mediated short hairpin RNA (shRNA) was designed to knockdown the expression of PP4R1 in ZR-75-30 breast cancer cells. The efficiency of lentivirus-mediated shRNA infection was determined using fluorescence microscopy to observe lentivirus-mediated green fluorescent protein expression and confirmed to be over 80%. PP4R1 expression in infected ZR-75-30 cells was detected by quantitative real-time polymerase chain reaction and western blot analysis. Cell proliferation and colony formation ability were measured by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay and colony formation assay, respectively. Flow cytometry was used to measure cell cycle progression and cell apoptosis. In addition, apoptosis makers, including poly-ADP-ribose polymerase (PARP) and caspase-3, were investigated in PP4R1-silenced ZR-75-30 cells by western blot assay.
RESULTS
We successfully constructed lentivirus-mediated shRNA to target PP4R1 in ZR-75-30 cells. MTT assay and colony formation assay showed the loss of PP4R1 suppressed the proliferation of ZR-75-30 cells. Flow cytometry analysis indicated cell cycle arrest and increased cell apoptosis in PP4R1 knockdown cells. Further, the apoptosis response in cells depleted of PP4R1 was illustrated by downregulation of PARP and upregulation of caspase-3.
CONCLUSION
Our results suggest that PP4R1 could promote breast cancer cell proliferation and might play a vital role in breast cancer occurrence.
MeSH Terms
-
Apoptosis
Blotting, Western
Breast Neoplasms*
Breast*
Caspase 3
Catalytic Domain
Cell Cycle
Cell Cycle Checkpoints
Cell Proliferation
Down-Regulation
Flow Cytometry
Humans
Microscopy, Fluorescence
Population Characteristics
Real-Time Polymerase Chain Reaction
RNA*
RNA, Small Interfering
Up-Regulation
Caspase 3
RNA
RNA, Small Interfering
Figure
Reference
-
1. Li CI, Daling JR, Malone KE. Age-specific incidence rates of in situ breast carcinomas by histologic type, 1980 to 2001. Cancer Epidemiol Biomarkers Prev. 2005; 14:1008–1011.
Article2. Anderson BO, Yip CH, Ramsey SD, Bengoa R, Braun S, Fitch M, et al. Breast cancer in limited-resource countries: health care systems and public policy. Breast J. 2006; 12:Suppl 1. S54–S69.
Article3. Parkin DM, Fernández LM. Use of statistics to assess the global burden of breast cancer. Breast J. 2006; 12:Suppl 1. S70–S80.
Article4. Allgayer H, Fulda S. An introduction to molecular targeted therapy of cancer. Adv Med Sci. 2008; 53:130–138.
Article5. Inostroza J, Sáenz L, Calaf G, Cabello G, Parra E. Role of the phosphatase PP4 in the activation of JNK-1 in prostate carcinoma cell lines PC-3 and LNCaP resulting in increased AP-1 and EGR-1 activity. Biol Res. 2005; 38:163–178.
Article6. Mourtada-Maarabouni M, Williams GT. Protein phosphatase 4 regulatesapoptosis in leukemic and primary human T-cells. Leuk Res. 2009; 33:1539–1551.
Article7. Chen GI, Tisayakorn S, Jorgensen C, D'Ambrosio LM, Goudreault M, Gingras AC. PP4R4/KIAA1622 forms a novel stable cytosolic complex with phosphoprotein phosphatase 4. J Biol Chem. 2008; 283:29273–29284.
Article8. Wang B, Zhao A, Sun L, Zhong X, Zhong J, Wang H, et al. Protein phosphatase PP4 is overexpressed in human breast and lung tumors. Cell Res. 2008; 18:974–977.
Article9. Kloeker S, Wadzinski BE. Purification and identification of a novel subunit of protein serine/threonine phosphatase 4. J Biol Chem. 1999; 274:5339–5347.
Article10. Zhang X, Ozawa Y, Lee H, Wen YD, Tan TH, Wadzinski BE, et al. Histone deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine phosphatase 4. Genes Dev. 2005; 19:827–839.
Article11. Lin HC, Wu CL, Chen YL, Huang JS, Wong TY, Yuan K. High-level β1-integrin expression in a subpopulation of highly tumorigenic oral cancer cells. Clin Oral Investig. 2014; 18:1277–1284.
Article12. Ren W, Wang X, Gao L, Li S, Yan X, Zhang J, et al. MiR-21 modulates chemosensitivity of tongue squamous cell carcinoma cells to cisplatin by targeting PDCD4. Mol Cell Biochem. 2014; 390:253–262.
Article13. Wang Y, Yuan F. Delivery of viral vectors to tumor cells: extracellular transport, systemic distribution, and strategies for improvement. Ann Biomed Eng. 2006; 34:114–127.
Article14. Takeshita F, Ochiya T. Therapeutic potential of RNA interference against cancer. Cancer Sci. 2006; 97:689–696.
Article15. Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002; 296:550–553.
Article16. Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002; 2:243–247.
Article17. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001; 411:494–498.
Article18. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005; 11:5678–5685.
Article19. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–172.
Article20. Toyo-oka K, Mori D, Yano Y, Shiota M, Iwao H, Goto H, et al. Protein phosphatase 4 catalytic subunit regulates Cdk1 activity and microtubule organization via NDEL1 dephosphorylation. J Cell Biol. 2008; 180:1133–1147.
Article21. Lee DH, Pan Y, Kanner S, Sung P, Borowiec JA, Chowdhury D. A PP4 phosphatase complex dephosphorylates RPA2 to facilitate DNA repair via homologous recombination. Nat Struct Mol Biol. 2010; 17:365–372.
Article22. He PX, Che YS, He QJ, Chen Y, Ding J. G226, a novel epipolythiodioxopiperazine derivative, induces autophagy and caspase-dependent apoptosis in human breast cancer cells in vitro. Acta Pharmacol Sin. 2014; 35:1055–1064.
Article23. Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999; 17:2941–2953.
Article24. Bressenot A, Marchal S, Bezdetnaya L, Garrier J, Guillemin F, Plénat F. Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J Histochem Cytochem. 2009; 57:289–300.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retraction note to: Lentivirus-Mediated Short-Hairpin RNA Targeting Protein Phosphatase 4 Regulatory Subunit 1 Inhibits Growth in Breast Cancer
- Erratum: Lentivirus-Mediated Short-Hairpin RNA Targeting Protein Phosphatase 4 Regulatory Subunit 1 Inhibits Growth in Breast Cancer
- Lentivirus-mediated RNA interference targeting E2F-1 inhibits human gastric cancer MGC-803 cell growth in vivo
- Inhibition of in vitro hepatitis B virus replication by lentivirus-mediated short-hairpin RNA against HBx
- Effect of Small Hairpin RNA Molecules Targeting Angiotensin-converting Enzyme Gene in Spontaneously Hypertensive Rats