J Korean Soc Hypertens.
2012 Sep;18(3):105-116.
Effect of Small Hairpin RNA Molecules Targeting Angiotensin-converting Enzyme Gene in Spontaneously Hypertensive Rats
- Affiliations
-
- 1Department of Pediatrics, Ewha Womans University Mokdong Hospital, Ewha Womans University School of Medicine, Seoul, Korea. ymhong@ewha.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, Ewha Womans University Mokdong Hospital, Ewha Womans University School of Medicine, Seoul, Korea.
Abstract
- BACKGROUND
Interfering RNA (iRNA) represents a recent breakthrough in effective blocking of the target genes in mammalian cells. Angiotensin-converting enzyme (ACE) has been shown to play an important role in the pathogenesis of hypertension. The purposes of this study were to investigate the effects on blood pressure, myocardial hypertrophy and gene expressions of iRNA targeting ACE.
METHODS
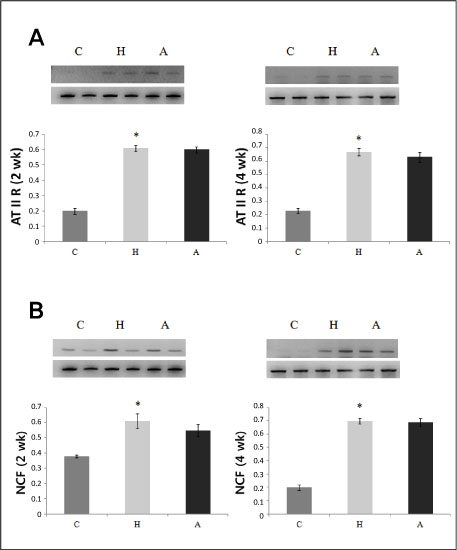
Twelve week old male Wistar-Kyoto rats were grouped as follows: control group (C group), spontaneously hypertensive rat (SHR) group (H group), and ACE-iRNA group (A group) in which SHR was treated with recombinant lentiviral vectors carrying small hairpin RNA targeting ACE. Reverse transcription-polymerase chain reaction and western blot analysis of ACE, endothelin (ET)-1, angiotensin (AT) II receptor type 1A, neutrophil cytosolic factor, caspase 3, Bax, and Bcl-2 were performed in the heart tissues. Serum AT, ACE, and high sensitive-C reactive protein were estimated.
RESULTS
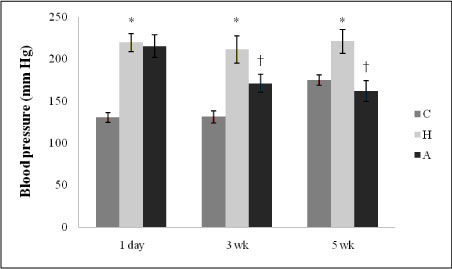
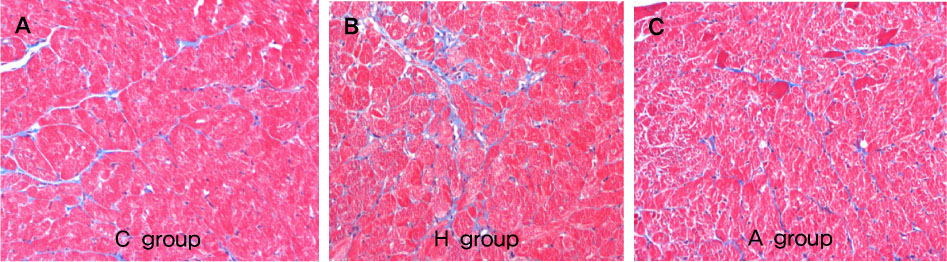
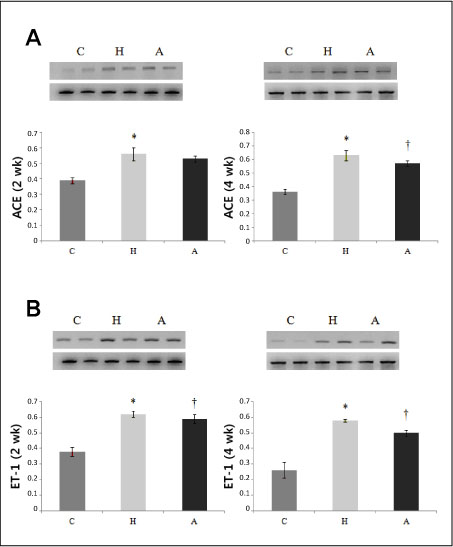
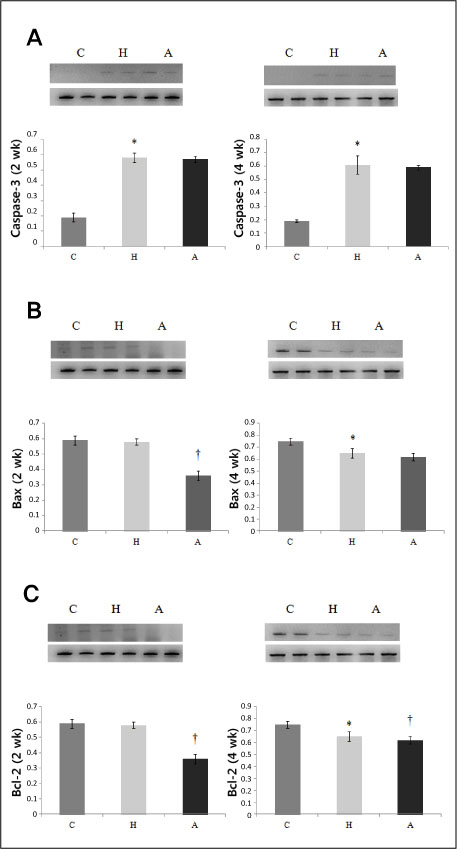
Systolic blood pressure was significantly decreased in the A group compared with the H group in weeks 3 and 5. Serum AT level was significantly lower on day 1, weeks 3 and 5 after ACE-iRNA treatment. ACE protein contents were significantly lower after ACE-iRNA treatment in week 5. ET-1 and Bcl-2 protein contents were significantly lower after ACE-iRNA treatment in weeks 3 and 5. Bax protein contents were significantly lower after ACE-iRNA treatment in week 3.
CONCLUSIONS
Recombinant lentiviral vectors carrying shRNA targeting ACE prevented hypertension. Serum AT and gene expressions such as ACE, ET-1, Bax, and Bcl-2 were significantly decreased after ACE-iRNA treatment.
Keyword
MeSH Terms
-
Angiotensins
Animals
bcl-2-Associated X Protein
Blood Pressure
Blotting, Western
Caspase 3
Cytosol
Endothelins
Gene Expression
Heart
Humans
Hypertension
Hypertrophy
Lentivirus
Lifting
Male
Neutrophils
Rats
Rats, Inbred SHR
RNA
RNA Interference
RNA, Small Interfering
Angiotensins
Caspase 3
Endothelins
RNA
RNA, Small Interfering
bcl-2-Associated X Protein
Figure
Reference
-
1. Matzke M, Matzke AJ, Kooter JM. RNA: guiding gene silencing. Science. 2001. 293:1080–1083.
Article2. Hannon GJ. RNA interference. Nature. 2002. 418:244–251.
Article3. Zamore PD. RNA interference: big applause for silencing in Stockholm. Cell. 2006. 127:1083–1086.
Article4. Escors D, Breckpot K. Lentiviral vectors in gene therapy: their current status and future potential. Arch Immunol Ther Exp (Warsz). 2010. 58:107–119.
Article5. Trittibach P, Barker SE, Broderick CA, Natkunarajah M, Duran Y, Robbie SJ, et al. Lentiviral-vector-mediated expression of murine IL-1 receptor antagonist or IL-10 reduces the severity of endotoxin-induced uveitis. Gene Ther. 2008. 15:1478–1488.
Article6. Jazwa A, Jozkowicz A, Dulak J. New vectors and strategies for cardiovascular gene therapy. Curr Gene Ther. 2007. 7:7–23.
Article7. Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011. 57:355–362.
Article8. Ferreira AJ, Murca TM, Fraga-Silva RA, Castro CH, Raizada MK, Santos RA. New cardiovascular and pulmonary therapeutic strategies based on the angiotensin-converting enzyme 2/angiotensin-(1-7)/mas receptor axis. Int J Hypertens. 2012. 2012:147825.
Article9. Gunaruwan P, Maher A, Williams L, Sharman J, Schmitt M, Campbell R, et al. Effects of bradykinin on venous capacitance in health and treated chronic heart failure. Clin Sci (Lond). 2009. 116:443–450.
Article10. Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med. 2009. 169:1195–1202.11. Martinez MV, Matulevicius S, Chin K. The association of right ventricular function and pulmonary arterial compliance in patients with idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2012. 59:13 Suppl. E1605.12. Arif H, Aijaz B, Islam M, Aftab U, Kumar S, Shafqat S. Drug compliance after stroke and myocardial infarction: a comparative study. Neurol India. 2007. 55:130–135.
Article13. Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, et al. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001. 107:465–476.
Article14. Freeman EJ, Ferrario CM, Tallant EA. Angiotensins differentially activate phospholipase D in vascular smooth muscle cells from spontaneously hypertensive and Wistar-Kyoto rats. Am J Hypertens. 1995. 8:1105–1111.
Article15. Lopez B, Gonzalez A, Varo N, Laviades C, Querejeta R, Diez J. Biochemical assessment of myocardial fibrosis in hypertensive heart disease. Hypertension. 2001. 38:1222–1226.
Article16. An SJ, Boyd R, Wang Y, Qiu X, Wang HD. Endothelin-1 expression in vascular adventitial fibroblasts. Am J Physiol Heart Circ Physiol. 2006. 290:H700–H708.
Article17. Yokoyama H, Averill DB, Brosnihan KB, Smith RD, Schiffrin EL, Ferrario CM. Role of blood pressure reduction in prevention of cardiac and vascular hypertrophy. Am J Hypertens. 2005. 18:922–929.
Article18. Fortuno MA, Ravassa S, Etayo JC, Diez J. Overexpression of Bax protein and enhanced apoptosis in the left ventricle of spontaneously hypertensive rats: effects of AT1 blockade with losartan. Hypertension. 1998. 32:280–286.19. Fortuno MA, Zalba G, Ravassa S, D'Elom E, Beaumont FJ, Fortuno A, et al. p53-mediated upregulation of BAX gene transcription is not involved in Bax-alpha protein overexpression in the left ventricle of spontaneously hypertensive rats. Hypertension. 1999. 33:1348–1352.20. Cha JH, Lee HR, Kim KC, Cho MS, Hong YM. Changes of gene expressions in spontaneously hypertensive rat model after losartan treatment. Korean Circ J. In press 2012.
Article21. He J, Bian Y, Gao F, Li M, Qiu L, Wu W, et al. RNA interference targeting the ACE gene reduced blood pressure and improved myocardial remodelling in SHRs. Clin Sci (Lond). 2009. 116:249–255.
Article22. Vazquez J, Correa de Adjounian MF, Sumners C, Gonzalez A, Diez-Freire C, Raizada MK. Selective silencing of angiotensin receptor subtype 1a (AT1aR) by RNA interference. Hypertension. 2005. 45:115–119.23. Phillips MI, Mohuczy-Dominiak D, Coffey M, Galli SM, Kimura B, Wu P, et al. Prolonged reduction of high blood pressure with an in vivo, nonpathogenic, adeno-associated viral vector delivery of AT1-R mRNA antisense. Hypertension. 1997. 29(1 Pt 2):374–380.24. Puddu GM, Cravero E, Ferrari E, Muscari A, Puddu P. Gene-based therapy for hypertension: do preclinical data suggest a promising future? Cardiology. 2007. 108:40–47.25. Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009. 105:724–736.
Article26. Shim TJ, Choi EJ, Park KJ, Kim YJ, Kim SJ, Lee SY, et al. Establishment of a eNOS gene therapy model using PLGA nanosphere in spontaneous hypertensive rat. J Korean Soc Hypertens. 2010. 16:28–38.27. Kim SJ, Kim MG, Choue CW, Kim KS, Song JS, Bae JH, et al. Association of angiotensin converting enzyme, angiotensinogen, and angiotensin II type 1 receptor gene polymorphisms with left ventricular hypertrophy in Korean hypertensive patients. J Korean Soc Hypertens. 2005. 11:44–52.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Angiotensin Converting Enzyme Inhibitors on Induced Angiotensin Converting Enzyme Activity in Rat Intestine
- Effect of Small Hairpin RNA Targeting Endothelin-Converting Enzyme-1 in Monocrotaline-Induced Pulmonary Hypertensive Rats
- Antihypertensive Properties of Dried Radish Leaves Powder in Spontaneously Hypertensive Rats
- Studies on the structural changes of aortic media and its repairing effect by enalapril in spontaneously hypertensive rats
- Angiotensin Converting Enzyme Inhibitors for the