Lab Anim Res.
2017 Jun;33(2):179-186. 10.5625/lar.2017.33.2.179.
Comparative study of fertilization rates of C57BL/6NKorl and C57BL/6N mice obtained from two other sources
- Affiliations
-
- 1College of Natural Resources and Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Department of Pharmacy, College of Pharmacy, Pusan National University, Busan, Korea.
- 3Departmentof Microbiology and Immunology, Inje University College of Medicine, Busan, Korea.
- 4Department of Health and Exercise Science, Korea National Sport University, Seoul, Korea.
- 5College of Veterinary Medicine, Kyungpook National University, Daegu, Korea.
- KMID: 2407433
- DOI: http://doi.org/10.5625/lar.2017.33.2.179
Abstract
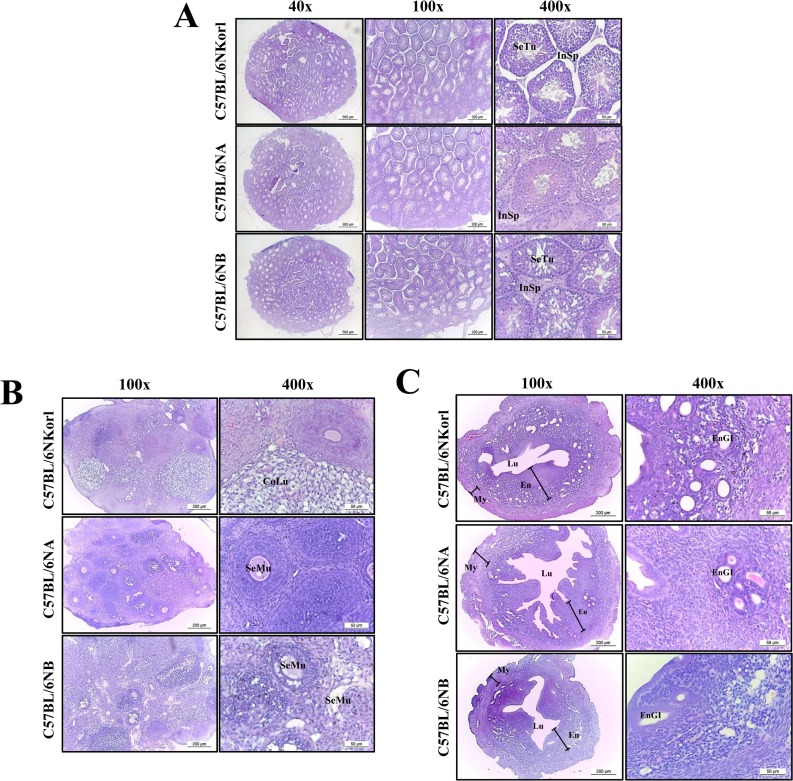
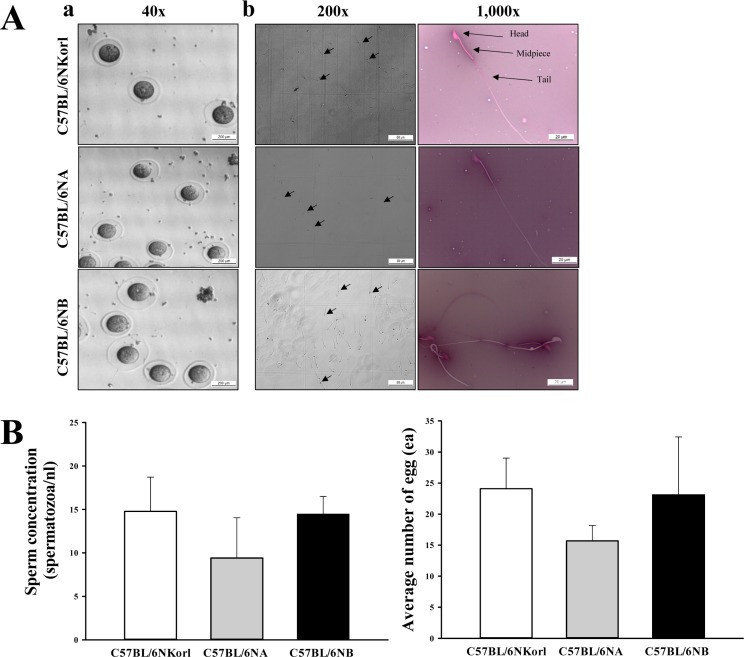
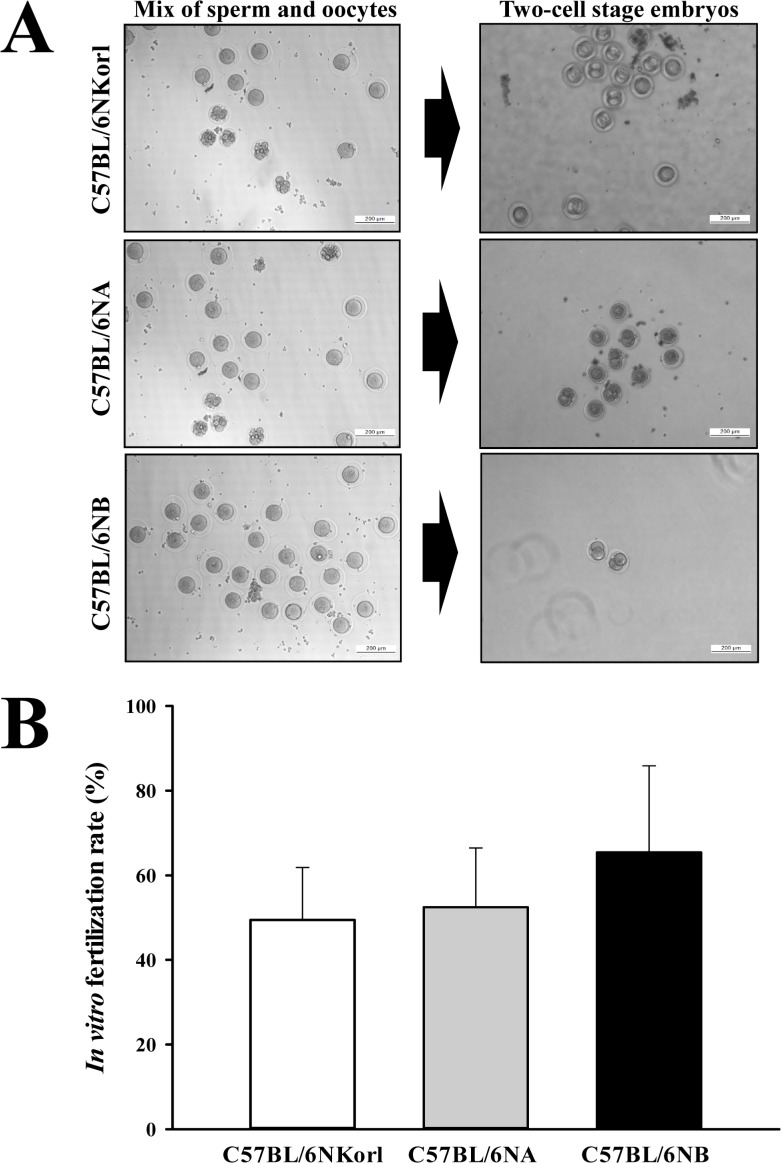
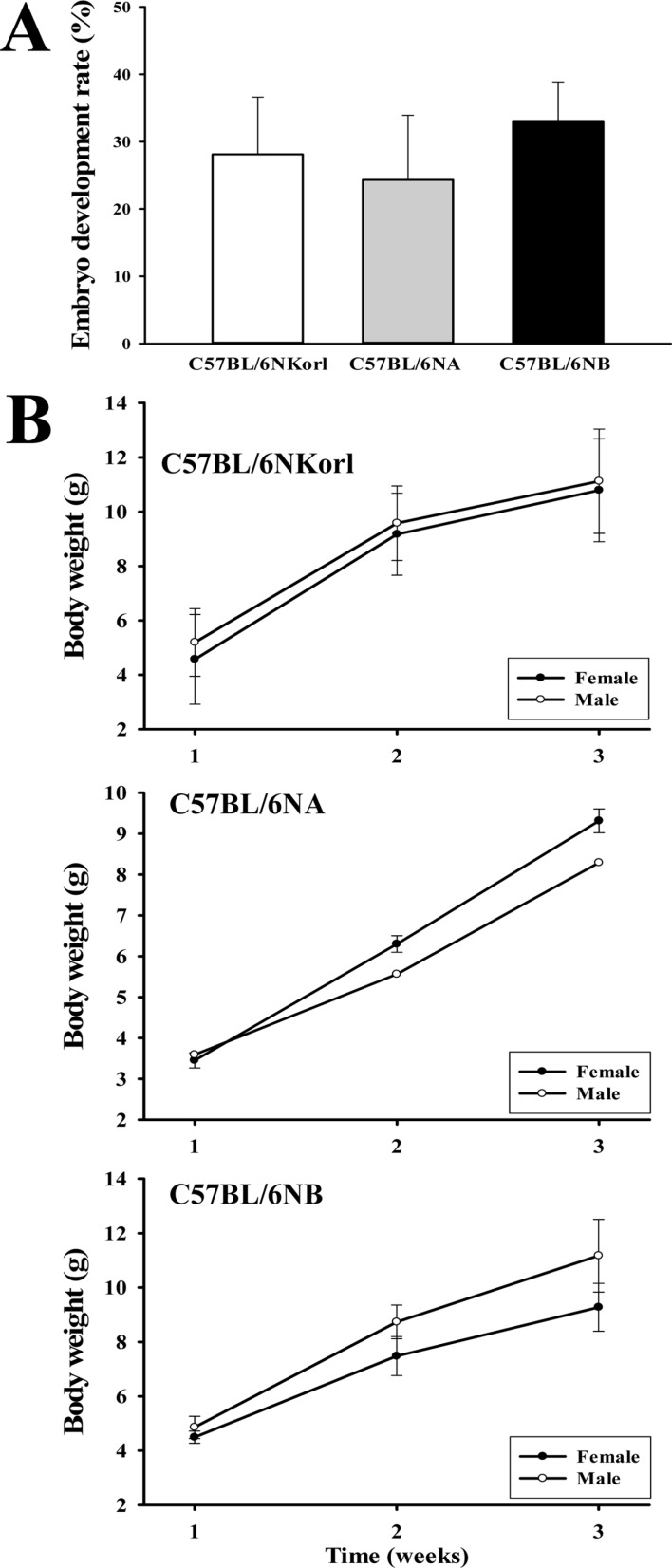
- C57BL/6N is the most widely used inbred mouse strain applied in a wide variety of research areas including cancer, cardiovascular biology, developmental biology, diabetes and obesity, genetics, immunology, neurobiology, and sensorineural research. To compare the fertilization rates of C57BL/6NKorl mice with two commercial C57BL/6N stocks, differences in reproductive organ structures, sperm and egg numbers, fertilization rates, and embryo development rates among C57BL/6NKorl (Korea FDA source), C57BL/6NA (USA source), and C57BL/6NB (Japan source) mice were determined. Among the stocks, no significant differences were detected in organ weight and histological structure of male and female reproductive organs, although body weight was higher in C57BL/6NKorl mice than that in the other groups. The concentration and morphology of sperm and eggs in C57BL/6NKorl mice were similar to those of C57BL/6NA and C57BL/6NB mice. Furthermore, the three stocks had similar in vitro fertilization and embryo development rates, although these rates tended to be higher in C57BL/6NB mice. Pup body weight was higher in C57BL/6NKorl and C57BL/6NB mice than that in C57BL/6NA mice. The results of the present study suggest that C57BL/6NKorl, C57BL/6NA, and C57BL/6NB mice obtained from three different sources have similar fertilization and embryo development rates, although there were slight differences in the magnitude of their responses rates.
Keyword
MeSH Terms
Figure
Reference
-
1. Hazzard KC, Watkins-Chow DE, Garrett LJ. Method of euthanasia influences the oocyte fertilization rate with fresh mouse sperm. J Am Assoc Lab Anim Sci. 2014; 53(6):641–646. PMID: 25650969.2. Marschall S, Huffstadt U, Balling R, Hrab de Angelis M. Reliable recovery of inbred mouse lines using cryopreserved spermatozoa. Mamm Genome. 1999; 10(8):773–776. PMID: 10430662.
Article3. Sohn C, Chang YS. Study on in vitro fertilization of mouse. Seoul J Med. 1985; 26(4):361–368.4. Laufer N, Pratt BM, DeCherney AH, Naftolin F, Merino M, Markert CL. The in vivo and in vitro effects of clomiphene citrate on ovulation, fertilization, and development of cultured mouse oocytes. Am J Obstet Gynecol. 1983; 147(6):633–639. PMID: 6638108.5. Iwamatsu T, Chang MC. Factors involved in the fertilization of mouse eggs in vitro. J Reprod Fertil. 1971; 26(2):197–208. PMID: 5105157.6. Hunter RH, Petersen HH, Greve T. Ovarian follicular fluid, progesterone and Ca2+ ion influences on sperm release from the fallopian tube reservoir. Mol Reprod Dev. 1999; 54(3):283–291. PMID: 10497350.7. Hansen GM, Markesich DC, Burnett MB, Zhu Q, Dionne KM, Richter LJ, Finnell RH, Sands AT, Zambrowicz BP, Abuin A. Large-scale gene trapping in C57BL/6N mouse embryonic stem cells. Genome Res. 2008; 18(10):1670–1679. PMID: 18799693.
Article8. Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, McRoberts JA. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J Neurogenet. 2008; 22(4):315–331. PMID: 19085272.
Article9. Rogner UC, Avner P. Congenic mice: cutting tools for complex immune disorders. Nat Rev Immunol. 2003; 3(3):243–252. PMID: 12658272.
Article10. Atochina EN, Beers MF, Tomer Y, Scanlon ST, Russo SJ, Panettieri RA Jr, Haczku A. Attenuated allergic airway hyperresponsiveness in C57BL/6 mice is associated with enhanced surfactant protein (SP)-D production following allergic sensitization. Respir Res. 2003; 4:15. PMID: 14748931.
Article11. Zurita E, Chagoyen M, Cantero M, Alonso R, González-Neira A, López-Jiménez A, López-Moreno JA, Landel CP, Benítez J, Pazos F, Montoliu L. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011; 20(3):481–489. PMID: 20506040.
Article12. Willott JF. Audition. In : Crusio WE, Sluyter F, Gerlai RT, Pietropaolo S, editors. Behavioral Genetics of the Mouse: Genetics of Behavioral Phenotypes. Cambridge: Cambridge University Press;2013. p. 36–44.13. Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999; 80(1-2):67–82. PMID: 10204719.
Article14. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization;2010. p. 32–44.15. Golshan Iranpour F, Rezazadeh Valojerdi M. The epididymal sperm viability, motility and DNA integrity in dead mice maintained at 4-6℃. Iran J Reprod Med. 2013; 11(3):195–200. PMID: 24639746.16. Park YS, Lee HJ, Seo JT, Lee YS, Hong JY, Lee HT, Chung KS. Effect of staining method on spermatozoa morphology. J Korean Androl Soc. 1994; 12(2):93–99.17. Liu L, Nutter LM, Law N, McKerlie C. Sperm freezing and in vitro fertilization in three substrains of C57BL/6 mice. J Am Assoc Lab Anim Sci. 2009; 48(1):39–43. PMID: 19245749.18. Takahashi H, Liu C. Archiving and distributing mouse lines by sperm cryopreservation, IVF, and embryo transfer. Methods Enzymol. 2010; 476:53–69. PMID: 20691860.
Article19. Germond M, Nocera D, Senn A, Rink K, Delacretaz G, Pedrazzini T, Hornung JP. Improved fertilization and implantation rates after non-touch zona pellucida microdrilling of mouse oocytes with a 1.48 microm diode laser beam. Hum Reprod. 1996; 11(5):1043–1048. PMID: 8671388.20. Kent GC. Comparative Anatomy of the Vertebrates. 9th Ed. Boston: McGraw Hill Higher Education;2001. p. 1–544.21. Giwercman A, Giwercman YL. Environmental factors and testicular function. Best Pract Res Clin Endocrinol Metab. 2011; 25(2):391–402. PMID: 21397206.
Article22. Taberlet P, Coissac E, Pansu J, Pompanon F. Conservation genetics of cattle, sheep, and goats. C R Biol. 2011; 334(3):247–254. PMID: 21377620.
Article23. Burns BM, Fordyce G, Holroyd RG. A review of factors that impact on the capacity of beef cattle females to conceive, maintain a pregnancy and wean a calf-Implications for reproductive efficiency in northern Australia. Anim Reprod Sci. 2010; 122(1-2):1–22. PMID: 20447780.
Article24. Yildiz C, Ottaviani P, Law N, Ayearst R, Liu L, McKerlie C. Effects of cryopreservation on sperm quality, nuclear DNA integrity, in vitro fertilization, and in vitro embryo development in the mouse. Reproduction. 2007; 133(3):585–595. PMID: 17379653.25. Vergara GJ, Irwin MH, Moffatt RJ, Pinkert CA. In vitro fertilization in mice: Strain differences in response to superovulation protocols and effect of cumulus cell removal. Theriogenology. 1997; 47(6):1245–1252. PMID: 16728073.26. Vasudevan K, Raber J, Sztein J. Fertility comparison between wild type and transgenic mice by in vitro fertilization. Transgenic Res. 2010; 19(4):587–594. PMID: 19844803.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of C57BL/6N mice on the variety of immunological researches
- Comparative analysis of restraint stress-induced depressive-like phenotypes in C57BL/6N mice derived from three different sources
- Comparison of humoral and cell-mediated immunity in three different C57BL/6N mouse substrains
- Comparative study of the immunological characteristics of three different C57BL/6N mouse substrains
- Erratum: Comparative study of fertilization rates of C57BL/6NKorl and C57BL/6N mice obtained from two other sources