J Korean Ophthalmol Soc.
2018 Mar;59(3):252-260. 10.3341/jkos.2018.59.3.252.
Effects of High Glucose and Dexamethasone on the Permeability in Trabecular Meshwork Cells
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu School of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2406960
- DOI: http://doi.org/10.3341/jkos.2018.59.3.252
Abstract
- PURPOSE
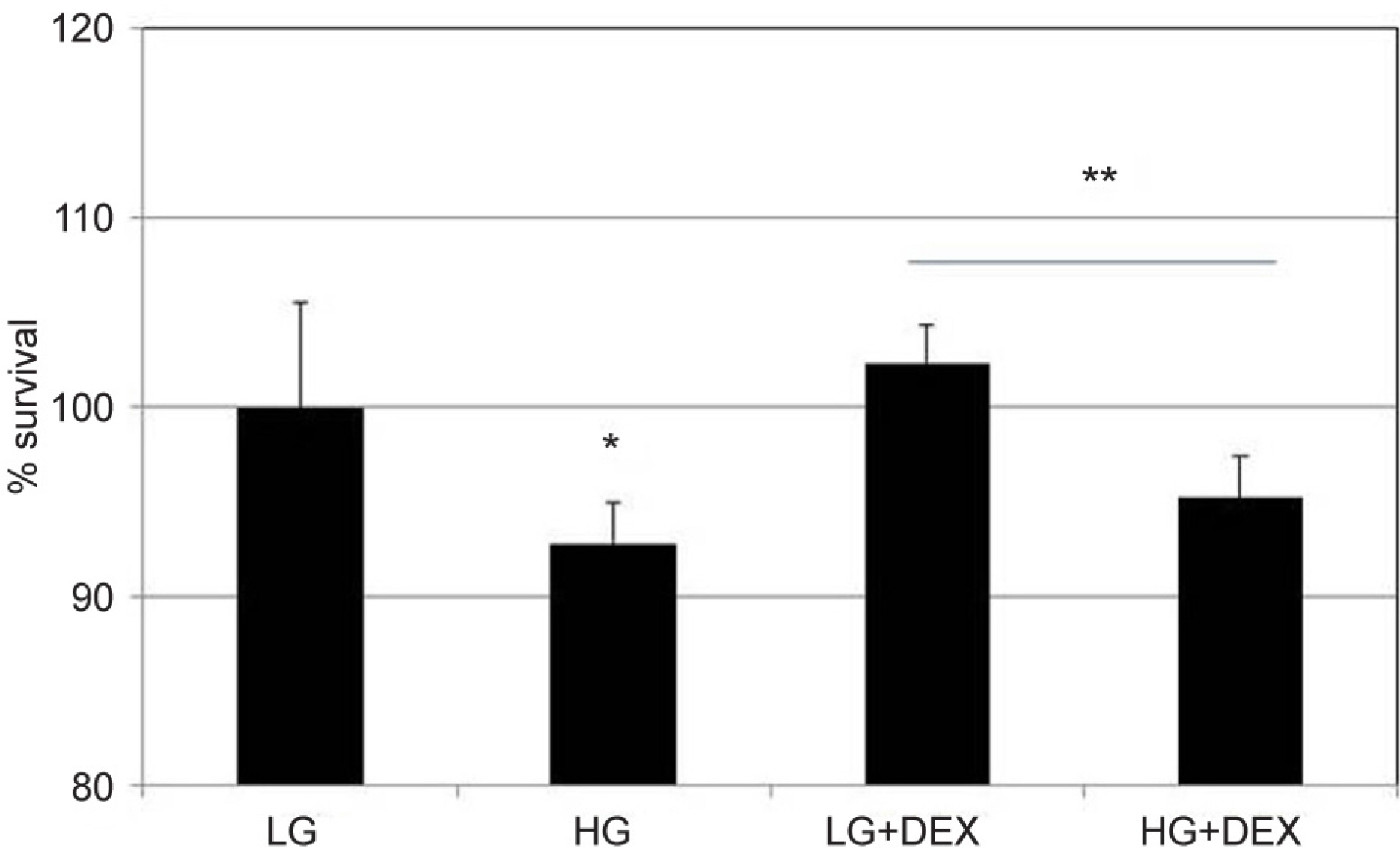
To investigate the effects of high glucose (HG) and dexamethasone (DEX) on the survival and permeability of trabecular meshwork cells (HTMC), and associated changes in tight junctions.
METHODS
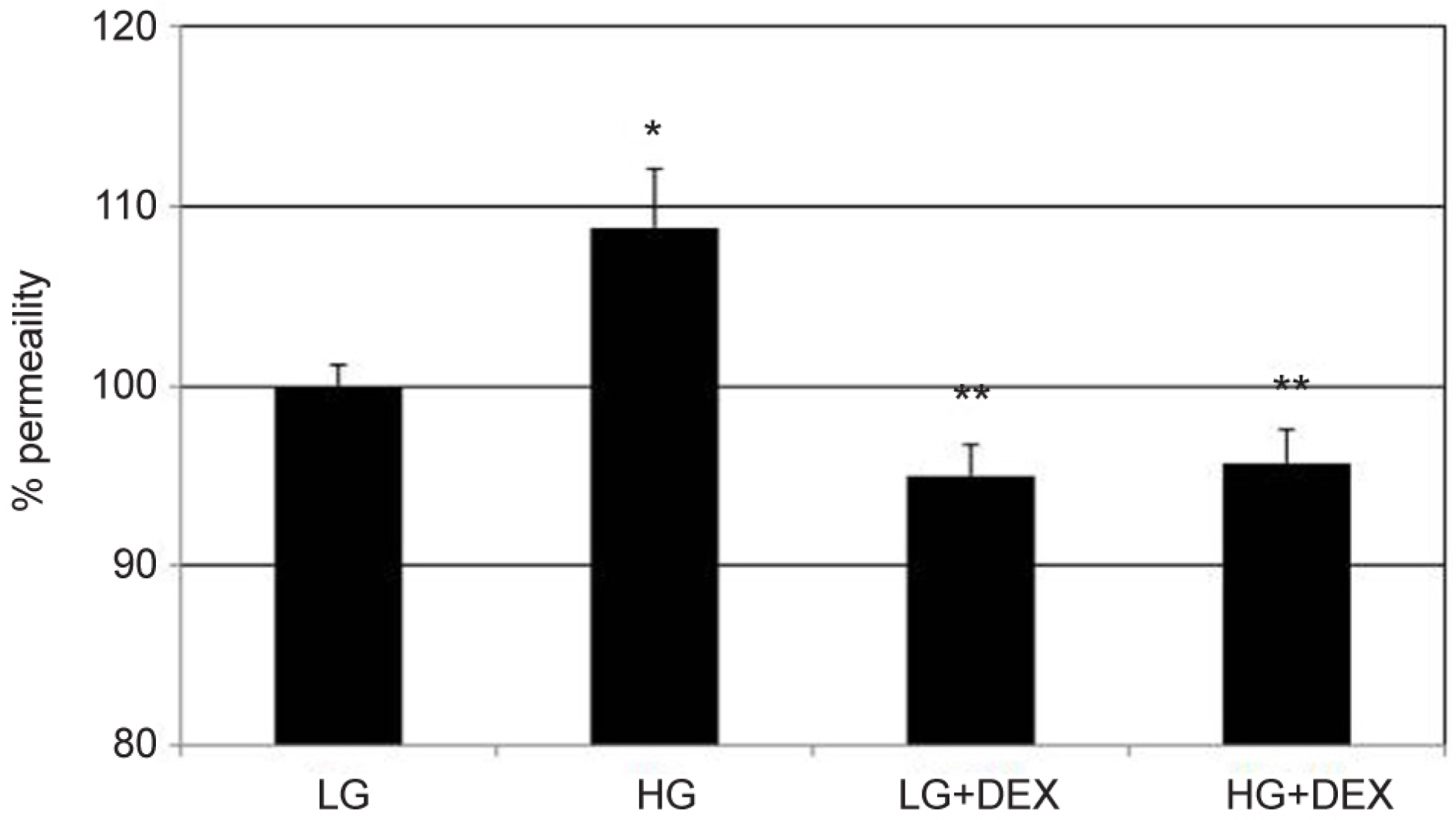
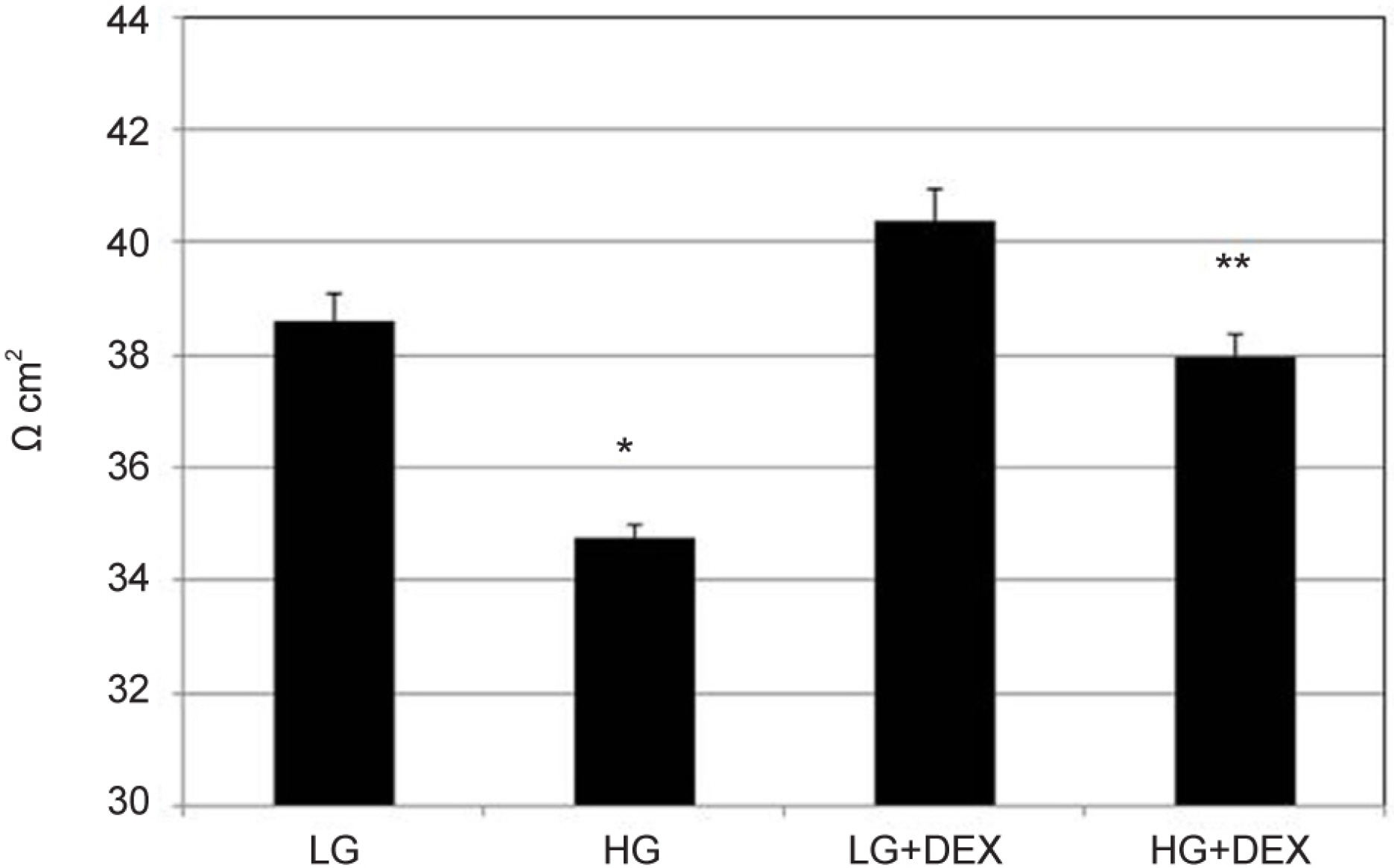
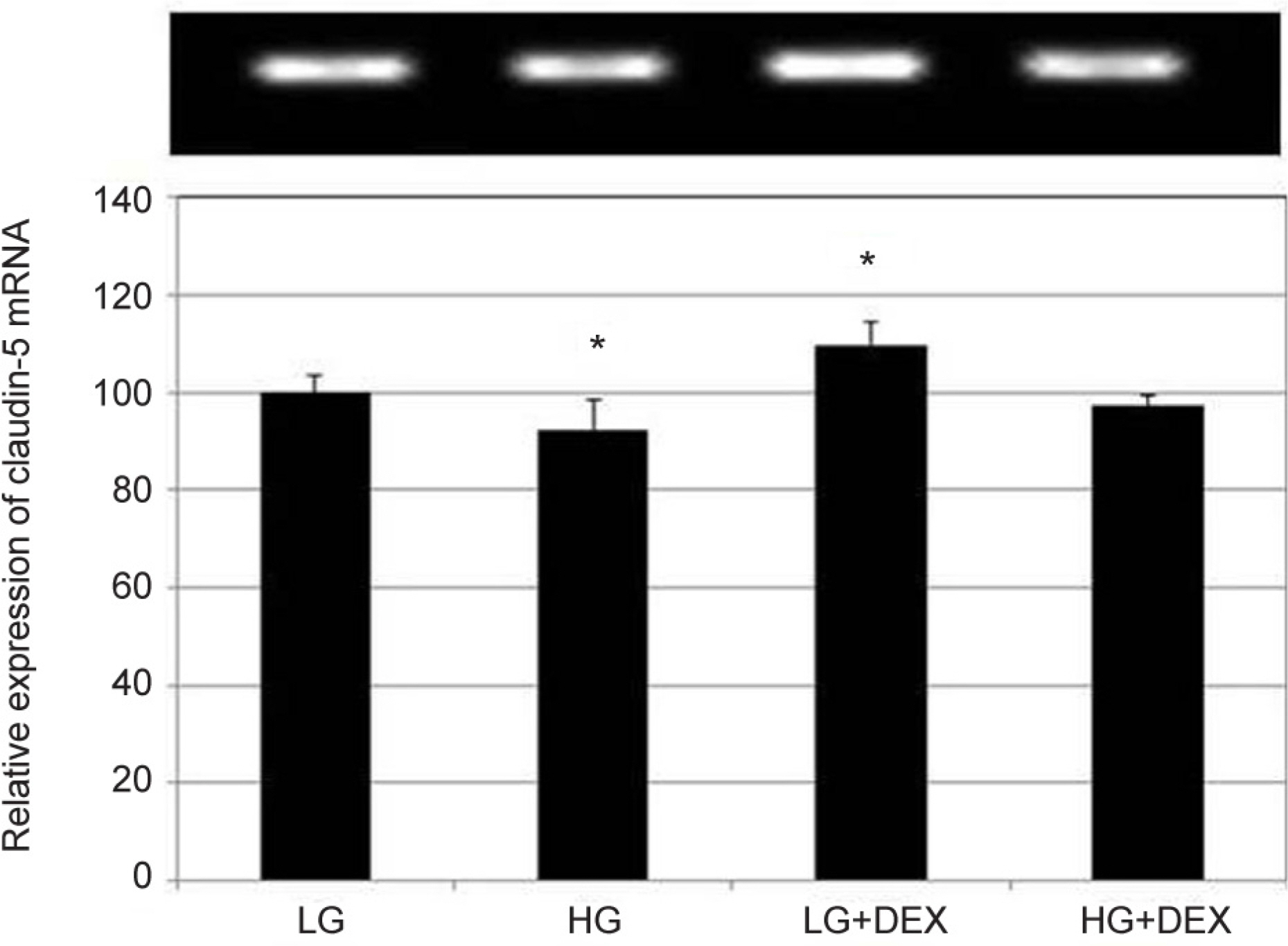
Primary cultured HTMC were exposed to 5 mM low glucose (LG) or 25 mM HG with or without 1.0 µM DEX for 3 days. The permeability of the HTMC monolayer was assessed using carboxyfluorescein or transendothelial electrical resistance (TEER). Gene and protein expressions of claudin-5 and occludin were assessed with reverse transcription polymerase chain reaction (RT-PCR) and Western blot, respectively.
RESULTS
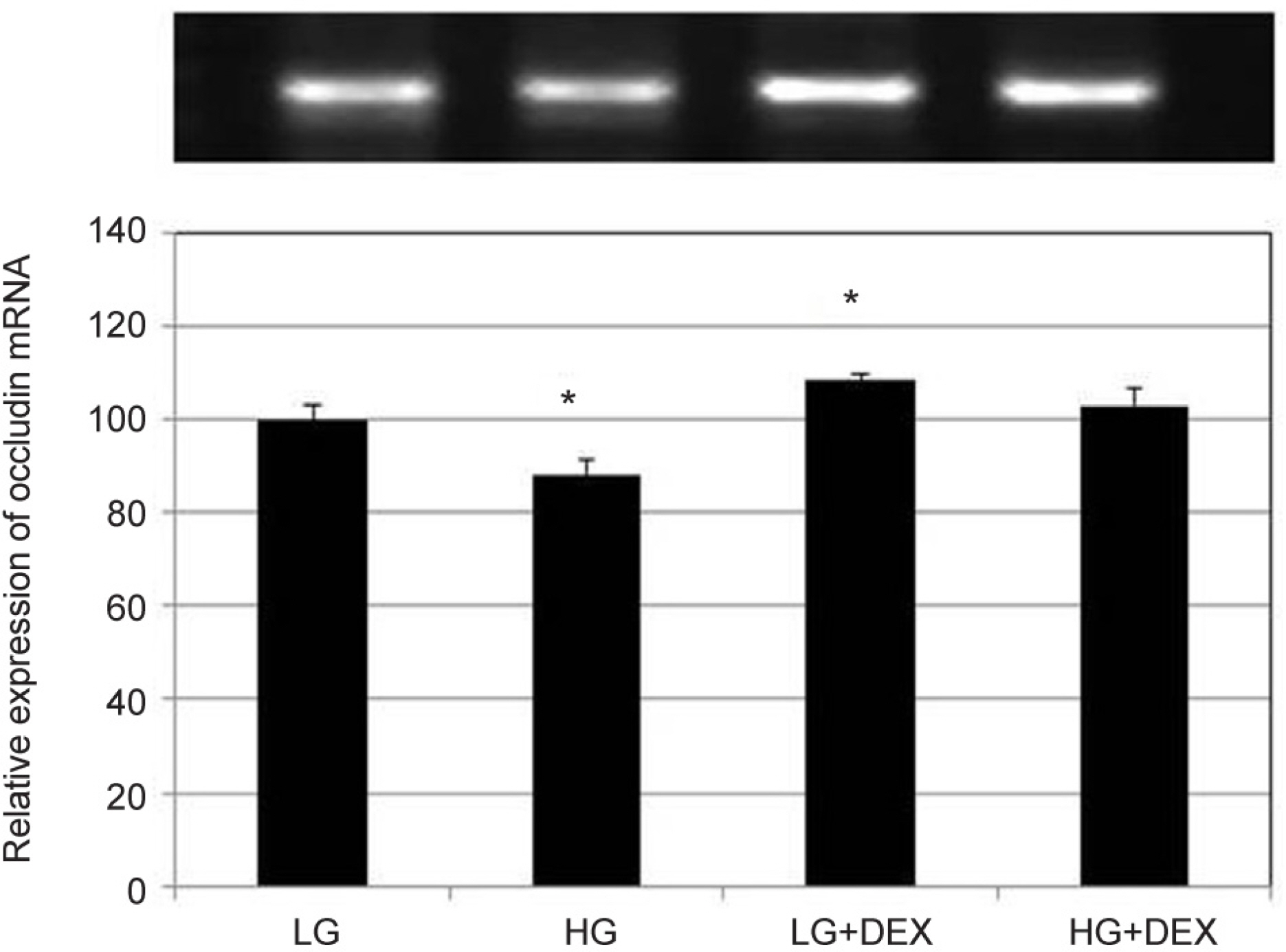
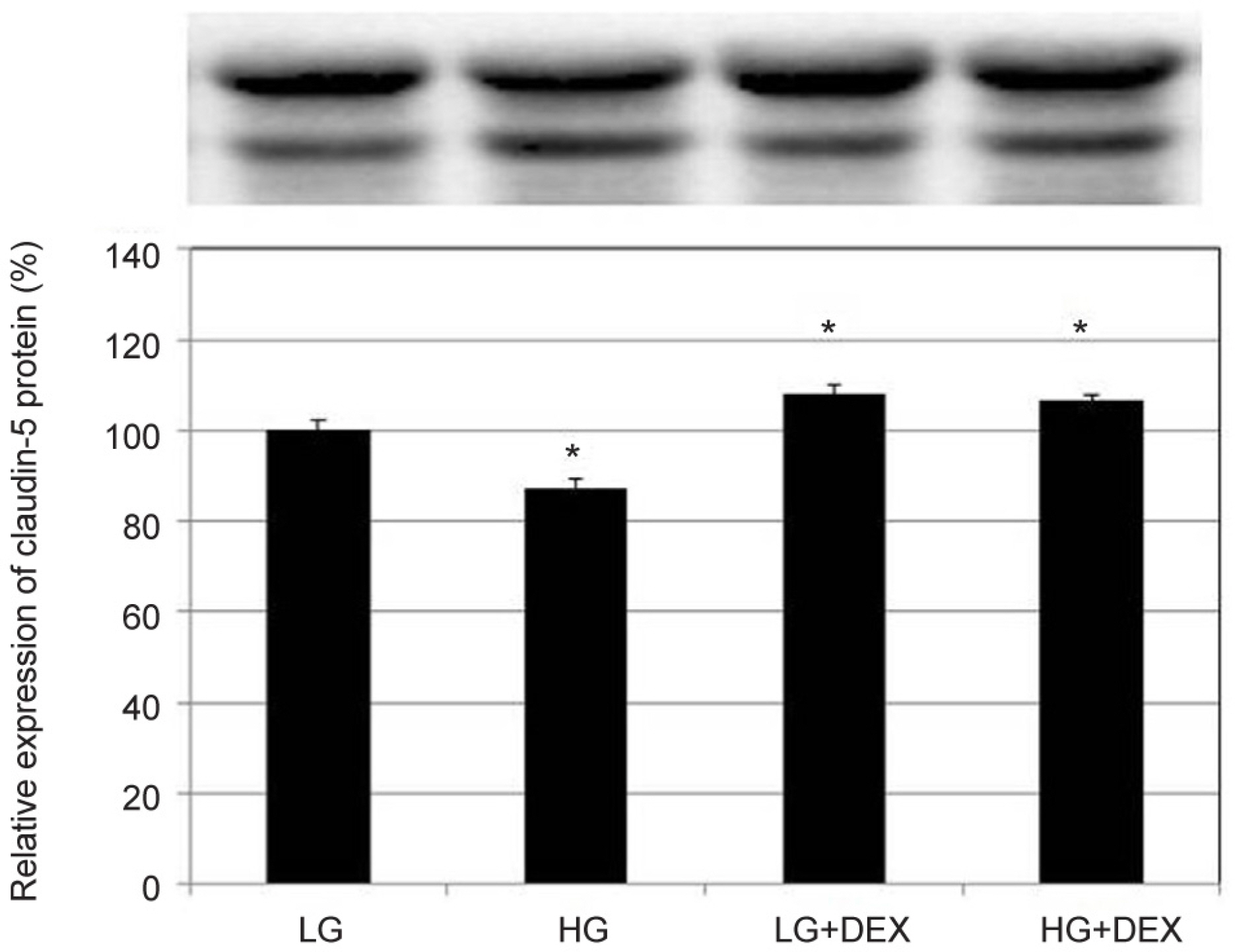
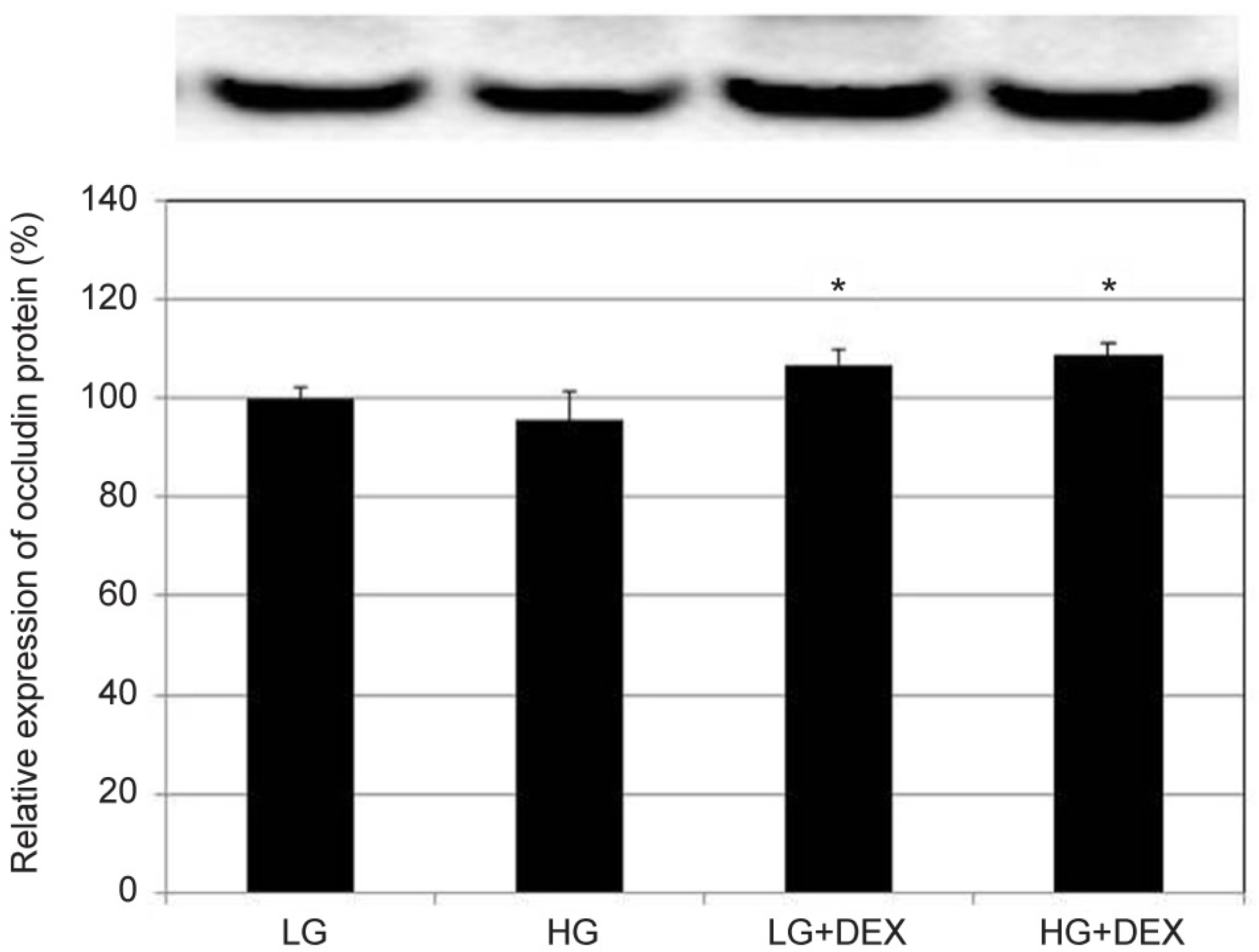
HG was significantly associated with greater HTMC monolayer permeability compared to LG by both the carboxyfluorescein permeability test and TEER (p = 0.022, 0.028). HG also decreased claudin-5 and occludin mRNA expression, respectively (7.5%, 12.9%). DEX abolished HG-induced increased permeability, and increased the protein expression of claudin-5 and occludin, respectively (p = 0.015, 0.012).
CONCLUSIONS
In HTMCs, DEX reversed HG-induced permeability increase. DEX increased tight junction molecules claudin-5 and occludin. Thus, DEX-induced changes in junctional proteins could be another mechanism of increased resistance through the trabecular meshwork and may result in steroid-induced glaucoma.
MeSH Terms
Figure
Reference
-
1). Becker B. Diabetes mellitus and primary open-angle glaucoma. The XXVII Edward Jackson Memorial Lecture. Am J Ophthalmol. 1971; 71(1 Pt 1):1–16.2). Davies PD, Duncan G, Pynsent PB, et al. Aqueous humour glucose concentration in cataract patients and its effect on the lens. Exp Eye Res. 1984; 39:605–9.
Article3). Lorenzi M, Cagliero E, Toledo S. Glucose toxicity for human endothelial cells in culture. Delayed replication, disturbed cell cycle, and accelerated death. Diabetes. 1985; 34:621–7.
Article4). Lorenzi M, Montisano DF, Toledo S, Barrieux A. High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest. 1986; 77:322–5.
Article5). Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema. Diabetes Care. 2003; 26:2653–64.
Article6). Mohr S. Potential new strategies to prevent the development of diabetic retinopathy. Expert Opin Invest Drugs. 2004; 13:189–98.
Article7). Antonetti DA, Barber AJ, Khin S, et al. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998; 47:1953–9.
Article8). Penfold PL, Wen L, Madigan MC, et al. Modulation of permeability and adhesion molecule expression by human choroidal endothelial cells. Invest Ophthalmol Vis Sci. 2002; 43:3125–30.9). Felinski EA, Antonetti DA. Glucocorticoid regulation of endothelial cell tight junction gene expression: novel treatments for diabetic retinopathy. Curr Eye Res. 2005; 30:949–57.
Article10). Saker S, Stewart EA, Browning AC, et al. The effect of hyperglycemia on permeability and the expression ofjunctional complex molecules in human retinal and choroidal endothelial cells. Exp Eye Res. 2014; 121:161–7.11). Stewart EA, Saker S, Amoaku WM. Dexamethasone reverses the effects of high glucose on human retinal endothelial cell permeability and proliferation in vitro. Exp Eye Res. 2016; 151:75–81.12). Wordinger RJ, Clark AF. Effects of glucocortocoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999; 18:629–67.13). François J. Corticosteroid glaucoma. Ann Ophthalmol. 1977; 9:1075–80.
Article14). Clark AF, Wilson K, McCartney MD, et al. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994; 35:281–94.15). Underwood JL, Murphy CG, Chen J, et al. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intercellular junctions. Am J Physiol. 1999; 277(2 Pt 1):C330–42.16). Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998; 60:121–42.
Article17). Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article18). Song J, Deng PF, Stinnett SS, et al. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci. 2005; 46:2424–32.
Article19). Kameda T, Inoue T, Inatani M, et al. The effect of Rho-associated protein kinase inhibitor on monkey Schlemm's canal endothelial cells. Invest Ophthalmol Vis Sci. 2012; 53:3092–103.
Article20). Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinasespecific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001; 42:1029–37.21). Alvarado JA, Betanzos A, Franse-Carman L, et al. Endothelia of Schlemm's canal and trabecular meshwork: distinct molecular, functional, and anatomic features. Am J Physiol Cell Physiol. 2004; 286:C621–34.
Article22). Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–9.23). Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–78.24). Partridge T, Jones GE, Gillett R. Cytochalasin B inhibits stabilisation of adhesions in fast-aggregating cell systems. Nature. 1975; 253:632–4.
Article25). Yun AJ, Murphy CG, Polansky JR, et al. Proteins secreted by human trabecular cells. Glucocorticoid and other effects. Invest Ophthalmol Vis Sci. 1989; 30:2012–22.26). Steely HT, Browder SL, Julian MB, et al. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1992; 33:242–50.27). O'Brien ET, Perkin SL, Roberts BC, Epstein DL. Dexamethasone inhibits trabecular cell retraction. Exp Eye Res. 1996; 62:675–88.28). Vitale S, Maguire MG, Murphy RP, et al. Clinically significant macular edema in type I diabetes. Incidence and risk factors. Ophthalmology. 1995; 102:1170–6.29). Levy J, Tovbin D, Lifshitz T, et al. Intraocular pressure during haemodialysis: a review. Eye (Lond). 2005; 19:1249–56.
Article30). Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995; 269(4 Pt 1):G467–75.
Article31). Balda MS, Whitney JA, Flores C, et al. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996; 134:1031–49.
Article32). Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993; 123(6 Pt 2):1777–88.
Article33). McCarthy KM, Skare IB, Stankewich MC, et al. Occludin is a functional component of the tight junction. J Cell Sci. 1996; 109(Pt 9):2287–98.
Article34). Lei Y, Stamer WD, Wu J, Sun X. Cell senescence reduced the mechanotransduction sensitivity of porcine angular aqueous plexus cells to elevation of pressure. Invest Ophthalmol Vis Sci. 2014; 55:2324–8.
Article35). Dejana E, Spagnuolo R, Bazzoni G. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb Haemost. 2001; 86:308–15.
Article36). Raghunathan VK, Morgan JT, Park SA, et al. Dexamethasone stiffens trabecular meshwork, trabecular meshwork cells, and matrix. Invest Ophthalmol Vis Sci. 2015; 56:4447–59.
Article37). Zhang X, Clark AF, Yorio T. Interactions of endothelin-1 with dexamethasone in primary cultured human trabeculae meshwork cells. Invest Ophthalmol Vis Sci. 2003; 44:5301–8.38). Clark AF, Steely HT, Dickerson JE Jr, et al. Glucocorticoid induction of the glaucoma gene MYOC in human and monkey trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2001; 42:1769–80.39). Hennis A, Wu SY, Nemesure B, et al. Hypertension, diabetes, and longitudinal changes in intraocular pressure. Ophthalmology. 2003; 110:908–14.
Article40). Dielemans I, de Jong PT, Stolk R, et al. Primary open-angle glaucoma, intraocular pressure, and diabetes mellitus in the general elderly population. The Rotterdam Study. Ophthalmology. 1996; 103:271–5.41). Mitchell P, Smith W, Chey T, Healey PR. Open-angle glaucoma and diabetes: the Blue Mountains Eye Study, Australia. Ophthalmology. 1997; 104:712–8.42). Zhao D, Cho J, Kim MH, et al. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology. 2015; 122:72–8.43). Tielsch JM, Katz J, Quigley HA, et al. Diabetes, intraocular pressure, and primary open-angle glaucoma in the Baltimore Eye Survey. Ophthalmology. 1995; 102:48–53.
Article44). Wormald RP, Basauri E, Wright LA, Evans JR. The African Caribbean Eye Survey: risk factors for glaucoma in a sample of African Caribbean people living in London. Eye (Lond). 1994; 8(Pt 3):315–20.
Article45). Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120:714–20. discussion 829-30.46). Gordon MO, Beiser JA, Kass MA. Is a history of diabetes mellitus protective against developing primary open-angle glaucoma? Arch Ophthalmol. 2008; 126:280–1.
Article47). Quigley HA. Can diabetes be good for glaucoma? Why can't we believe our own eyes (or data)? Arch Ophthalmol. 2009; 127:227–9.
Article48). Wiederholt M, Dorschner N, Groth J. Effect of diuretics, channel modulators and signal interceptors on contractility of the trabecular meshwork. Ophthalmologica. 1997; 211:153–60.
Article49). Wiederholt M, Stumpff F. The trabecular meshwork and aqueous humor reabsorption. Civan MM. Current Topics in Membranes. The Eye's Aqueous Humor: from Secretion to Glaucoma. 1st. San Diego: Academic Press;1998. v. 45. chap. 7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Benzalkonium, Mitomycin-C and Dexamethasone on Stress in Trabecular Meshwork Cells
- Effect of Bimatoprost on the Permeability of Trabecular Meshwork Cell Monolayer
- Effect of Tetrahydrozoline on the Permeability of Trabecular Meshwork Cell Monolayer
- Effect of High Glucose on the Production of Reactive Oxygen Species in Trabecular Meshwork Cells
- Effect of Latanoprostene Bunod on the Permeability of Trabecular Meshwork Cells