J Bone Metab.
2018 Feb;25(1):9-13. 10.11005/jbm.2018.25.1.9.
Trans-differentiation via Epigenetics: A New Paradigm in the Bone Regeneration
- Affiliations
-
- 1Department of Molecular Genetics, School of Dentistry and Dental Research Institute, BK21 Program, Seoul National University, Seoul, Korea. hmryoo@snu.ac.kr
- 2Department of Periodontology, School of Dentistry and Dental Research Institute, BK21 Program, Seoul National University, Seoul, Korea.
- KMID: 2406234
- DOI: http://doi.org/10.11005/jbm.2018.25.1.9
Abstract
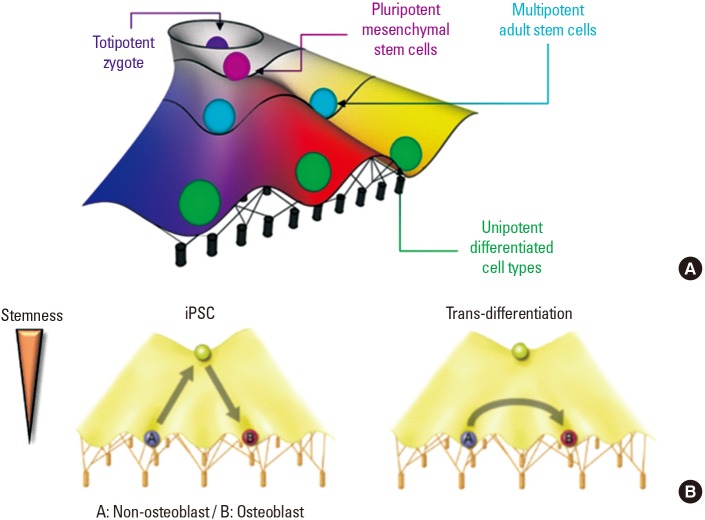
- In regenerative medicine, growing cells or tissues in the laboratory is necessary when damaged cells can not heal by themselves. Acquisition of the required cells from the patient's own cells or tissues is an ideal option without additive side effects. In this context, cell reprogramming methods, including the use of induced pluripotent stem cells (iPSCs) and trans-differentiation, have been widely studied in regenerative research. Both approaches have advantages and disadvantages, and the possibility of de-differentiation because of the epigenetic memory of iPSCs has strengthened the need for controlling the epigenetic background for successful cell reprogramming. Therefore, interest in epigenetics has increased in the field of regenerative medicine. Herein, we outline in detail the cell trans-differentiation method using epigenetic modification for bone regeneration in comparison to the use of iPSCs.
Keyword
MeSH Terms
Figure
Reference
-
1. Dimitriou R, Jones E, McGonagle D, et al. Bone regeneration: current concepts and future directions. BMC Med. 2011; 9:66. PMID: 21627784.
Article2. Henkel J, Woodruff MA, Epari DR, et al. Bone regeneration based on tissue engineering conceptions: a 21st century perspective. Bone Res. 2013; 1:216–248. PMID: 26273505.3. Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012; 481:295–305. PMID: 22258608.
Article4. Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012; 13:97–109. PMID: 22215131.
Article5. Dupont C, Armant DR, Brenner CA. Epigenetics: definition, mechanisms and clinical perspective. Semin Reprod Med. 2009; 27:351–357. PMID: 19711245.
Article6. Cho YD, Yoon WJ, Kim WJ, et al. Epigenetic modifications and canonical wingless/int-1 class (WNT) signaling enable trans-differentiation of nonosteogenic cells into osteoblasts. J Biol Chem. 2014; 289:20120–20128. PMID: 24867947.
Article7. Mondal T, Rasmussen M, Pandey GK, et al. Characterization of the RNA content of chromatin. Genome Res. 2010; 20:899–907. PMID: 20404130.
Article8. Gardiner-Garden M, Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987; 196:261–282. PMID: 3656447.
Article9. Kafri T, Ariel M, Brandeis M, et al. Developmental pattern of gene-specific DNA methylation in the mouse embryo and germ line. Genes Dev. 1992; 6:705–714. PMID: 1577268.
Article10. Bestor T, Laudano A, Mattaliano R, et al. Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. The carboxyl-terminal domain of the mammalian enzymes is related to bacterial restriction methyltransferases. J Mol Biol. 1988; 203:971–983. PMID: 3210246.11. Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011; 21:381–395. PMID: 21321607.
Article12. Shiota K. DNA methylation profiles of CpG islands for cellular differentiation and development in mammals. Cytogenet Genome Res. 2004; 105:325–334. PMID: 15237220.
Article13. Ohgane J, Yagi S, Shiota K. Epigenetics: the DNA methylation profile of tissue-dependent and differentially methylated regions in cells. Placenta. 2008; 29(Suppl A):S29–S35. PMID: 18031808.
Article14. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126:663–676. PMID: 16904174.
Article15. Medvedev SP, Shevchenko AI, Zakian SM. Induced pluripotent stem cells: problems and advantages when applying them in regenerative medicine. Acta Naturae. 2010; 2:18–28. PMID: 22649638.
Article16. Duan X, Tu Q, Zhang J, et al. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J Cell Physiol. 2011; 226:150–157. PMID: 20658533.
Article17. Hynes K, Menichanin D, Bright R, et al. Induced pluripotent stem cells: a new frontier for stem cells in dentistry. J Dent Res. 2015; 94:1508–1515. PMID: 26285811.18. Selman K, Kafatos FC. Transdifferentiation in the labial gland of silk moths: is DNA required for cellular metamorphosis? Cell Differ. 1974; 3:81–94. PMID: 4277742.
Article19. Eguchi G, Okada TS. Differentiation of lens tissue from the progeny of chick retinal pigment cells cultured in vitro: a demonstration of a switch of cell types in clonal cell culture. Proc Natl Acad Sci U S A. 1973; 70:1495–1499. PMID: 4576021.
Article20. Xu J, Du Y, Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell. 2015; 16:119–134. PMID: 25658369.
Article21. Cho YD, Bae HS, Lee DS, et al. Epigenetic priming confers direct cell trans-differentiation from adipocyte to osteoblast in a transgene-free state. J Cell Physiol. 2016; 231:1484–1494. PMID: 26335354.
Article22. Cho Y, Kim B, Bae H, et al. Direct gingival fibroblast/osteoblast transdifferentiation via epigenetics. J Dent Res. 2017; 96:555–561. PMID: 28081379.
Article23. Waddington CH. The strategy of the genes: A discussion of some aspects of theoretical biology. London, UK: Allen & Unwin;1957.24. Cho YD, Kim WJ, Yoon WJ, et al. Wnt3a stimulates Mepe, matrix extracellular phosphoglycoprotein, expression directly by the activation of the canonical Wnt signaling pathway and indirectly through the stimulation of autocrine Bmp-2 expression. J Cell Physiol. 2012; 227:2287–2296. PMID: 22213482.25. Graf T, Enver T. Forcing cells to change lineages. Nature. 2009; 462:587–594. PMID: 19956253.
Article26. Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011; 12:79–89. PMID: 21252997.
Article27. Xie H, Ye M, Feng R, et al. Stepwise reprogramming of B cells into macrophages. Cell. 2004; 117:663–676. PMID: 15163413.
Article28. Meivar-Levy I, Sapir T, Gefen-Halevi S, et al. Pancreatic and duodenal homeobox gene 1 induces hepatic dedifferentiation by suppressing the expression of CCAAT/enhancer-binding protein beta. Hepatology. 2007; 46:898–905. PMID: 17705277.29. Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010; 142:375–386. PMID: 20691899.
Article