J Dent Anesth Pain Med.
2018 Feb;18(1):9-17. 10.17245/jdapm.2018.18.1.9.
Effect of remifentanil on pre-osteoclast cell differentiation in vitro
- Affiliations
-
- 1Department of Dental Anesthesia and Pain Medicine, School of Dentistry, Pusan National University, Dental Research Institute, Yangsan, Republic of Korea. anekch@pusan.ac.kr
- 2Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Republic of Korea.
- 3Department of Anesthesia and Pain Medicine, Pusan National University Hospital, Busan, Republic of Korea.
- 4Department of Oral Physiology, School of Dentistry, Pusan National University, Yangsan, Republic of Korea.
- KMID: 2405378
- DOI: http://doi.org/10.17245/jdapm.2018.18.1.9
Abstract
- BACKGROUND
The structure and function of bone tissue is maintained through a constant remodeling process, which is maintained by the balance between osteoblasts and osteoclasts. The failure of bone remodeling can lead to pathological conditions of bone structure and function. Remifentanil is currently used as a narcotic analgesic agent in general anesthesia and sedation. However, the effect of remifentanil on osteoclasts has not been studied. Therefore, we investigated the effect of remifentanil on pre-osteoclast (pre-OCs) differentiation and the mechanism of osteoclast differentiation in the absence of specific stimulus.
METHODS
Pre-OCs were obtained by culturing bone marrow-derived macrophages (BMMs) in osteoclastogenic medium for 2 days and then treated with various concentration of remifentanil. The mRNA expression of NFATc1 and c-fos was examined by using real-time PCR. We also examined the effect of remifentanil on the osteoclast-specific genes TRAP, cathepsin K, calcitonin receptor, and DC-STAMP. Finally, we examined the influence of remifentanil on the migration of pre-OCs by using the Boyden chamber assay.
RESULTS
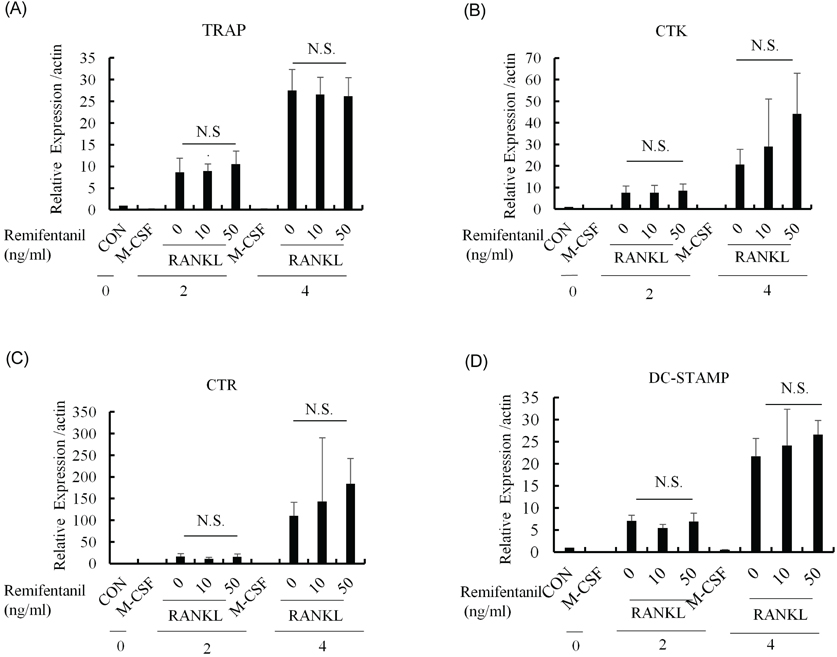
Remifentanil increased pre-OC differentiation and osteoclast size, but did not affect the mRNA expression of NFATc1 and c-fos or significantly affect the expression of TRAP, cathepsin K, calcitonin receptor, and DC-STAMP. However, remifentanil increased the migration of pre-OCs.
CONCLUSIONS
This study suggested that remifentanil promotes the differentiation of pre-OCs and induces maturation, such as increasing osteoclast size. In addition, the increase in osteoclast size was mediated by the enhancement of pre-OC migration and cell fusion.
Keyword
MeSH Terms
Figure
Reference
-
1. Chambers TJ. Regulation of the differentiation and function of osteoclasts. J Pathol. 2000; 192:4–13.
Article2. Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001; 16:1575–1582.
Article3. Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010; 285:25103–25108.
Article4. Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006; 1092:385–396.
Article5. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000; 289:1504–1508.
Article6. Roodman GD. Advances in bone biology: The osteoclast. Endocr Rev. 1996; 17:308–332.
Article7. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998; 93:165–176.
Article8. Chen EH, Grote E, Mohler W, Vignery A. Cell-cell fusion. FEBS Lett. 2007; 581:2181–2193.
Article9. Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003; 4:638–649.
Article10. Vignery A. Macrophage fusion: the making of osteoclasts and giant cells. J Exp Med. 2005; 202:337–340.11. Vignery A. Osteoclasts and giant cells: macrophagemacrophage fusion mechanism. Int J Exp Pathol. 2000; 81:291–304.
Article12. Glass PS, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg. 1999; 89:S7–S14.
Article13. Scott LJ, Perry CM. Remifentanil: A review of its use during the induction and maintenance of general anaesthesia. Drugs. 2005; 65:1793–1823.14. Yoon JY, Kim DW, Kim EJ, Park BS, Yoon JU, Kim HJ, et al. Protective effects of remifentanil against H2O2-induced oxidative stress in human osteoblasts. J Dent Anesth Pain Med. 2016; 16:263–271.
Article15. Baik SW, Park BS, Kim YH, Kim YD, Kim CH, Yoon JY, et al. Effects of remifentanil preconditioning on osteoblasts under hypoxia-reoxygenation condition. Int J Med Sci. 2015; 12:583–589.
Article16. Ha J, Choi HS, Lee Y, Lee ZH, Kim HH. Caffeic acid phenethyl ester inhibits osteoclastogenesis by suppressing NF kappaB and downregulating NFATc1 and c-Fos. Int Immunopharmacol. 2009; 9:774–780.
Article17. Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997; 15:707–747.18. Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002; 3:889–901.
Article19. Takayanagi H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med (Berl). 2005; 83:170–179.
Article20. Yagi M, Ninomiya K, Fujita N, Suzuki T, Iwasaki R, Morita K, et al. Induction of DC-STAMP by alternative activation and downstream signaling mechanisms. J Bone Miner Res. 2007; 22:992–1001.
Article21. Felix R, Hofstetter W, Wetterwald A, Cecchini MG, Fleisch H. Role of colony-stimulating factor-1 in bone metabolism. J Cell Biochem. 1994; 55:340–349.
Article22. Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, et al. c-Fos: A key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994; 266:443–448.
Article23. Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. 2012; 45:863–873.
Article24. Ishii M, Saeki Y. Osteoclast cell fusion: mechanisms and molecules. Mod Rheumatol. 2008; 18:220–227.
Article25. Corral DA, Amling M, Priemel M, Loyer E, Fuchs S, Ducy P, et al. Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proc Natl Acad Sci U S A. 1998; 95:13835–13840.
Article26. Hou GQ, Guo C, Song GH, Fang N, Fan WJ, Chen XD, et al. Lipopolysaccharide (LPS) promotes osteoclast differentiation and activation by enhancing the MAPK pathway and COX-2 expression in RAW264.7 cells. Int J Mol Med. 2013; 32:503–510.
Article27. Zou W, Bar-Shavit Z. Dual modulation of osteoclast differentiation by lipopolysaccharide. J Bone Miner Res. 2002; 17:1211–1218.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Remifentanil Negatively Regulates RANKL-Induced Osteoclast Differentiation and Bone Resorption by Inhibiting c-Fos/NFATc1 Expression
- Remifentanil promotes osteoblastogenesis by upregulating Runx2/osterix expression in preosteoblastic C2C12 cells
- Effect of Sonicated Extract of Treponema Denticola on Osteoclast Differentiation
- Poncirin Inhibits Osteoclast Differentiation and Bone Loss through Down-Regulation of NFATc1 In Vitro and In Vivo
- Odontogenic Ameloblast-Associated Protein (Odam) Plays Crucial Roles in Osteoclast Differentiation via Control of Actin Ring Formation