Cancer Res Treat.
2015 Oct;47(4):913-920. 10.4143/crt.2014.057.
A Novel, Potent, Small Molecule AKT Inhibitor Exhibits Efficacy against Lung Cancer Cells In Vitro
- Affiliations
-
- 1Department of Pharmacy, Birla Institute of Technology and Science, Pilani, Hyderabad Campus, Hyderabad, India.
- 2Incozen Therapeutics Pvt. Ltd., Hyderabad, India. srikantv@incozen.com
- KMID: 2403412
- DOI: http://doi.org/10.4143/crt.2014.057
Abstract
- PURPOSE
Anomalies of Akt regulation, including overexpression in lung cancer, impart resistance to conventional chemotherapy and radiation, thereby implicating this kinase as a therapeutic intervention point. A novel scaffold of Akt inhibitors was developed through virtual screening of chemical databases available at Birla Institute of Technology and Science, Pilani, Hyderabad, based on docking studies using Maestro. A benzothienopyrimidine derivative (BIA-6) was identified as a potential lead molecule that inhibited Akt1 enzyme activity with an IC50 of 256 nM.
MATERIALS AND METHODS
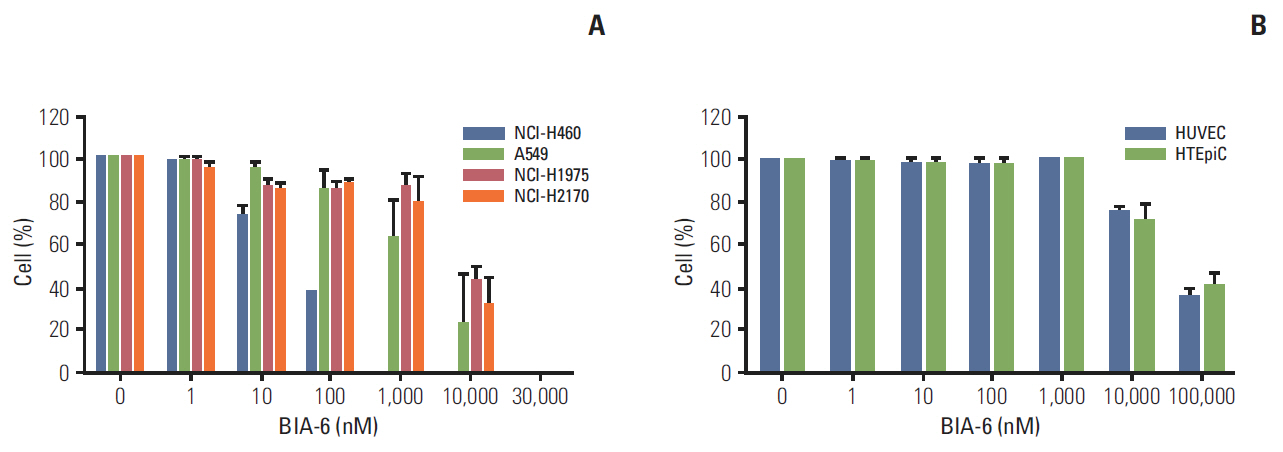
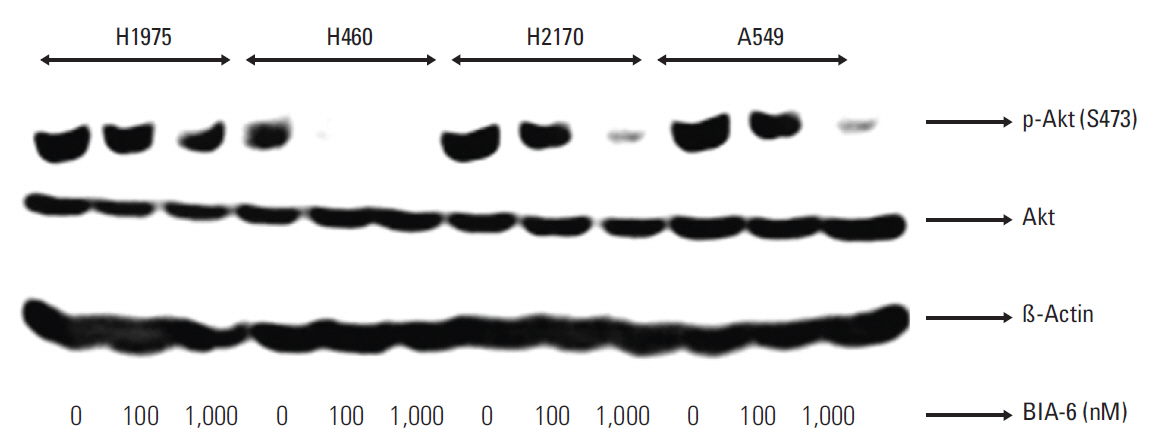
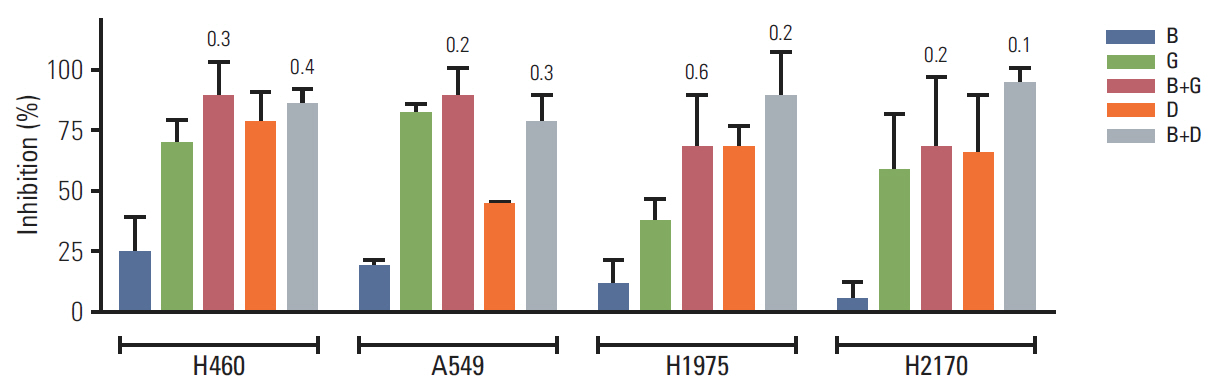
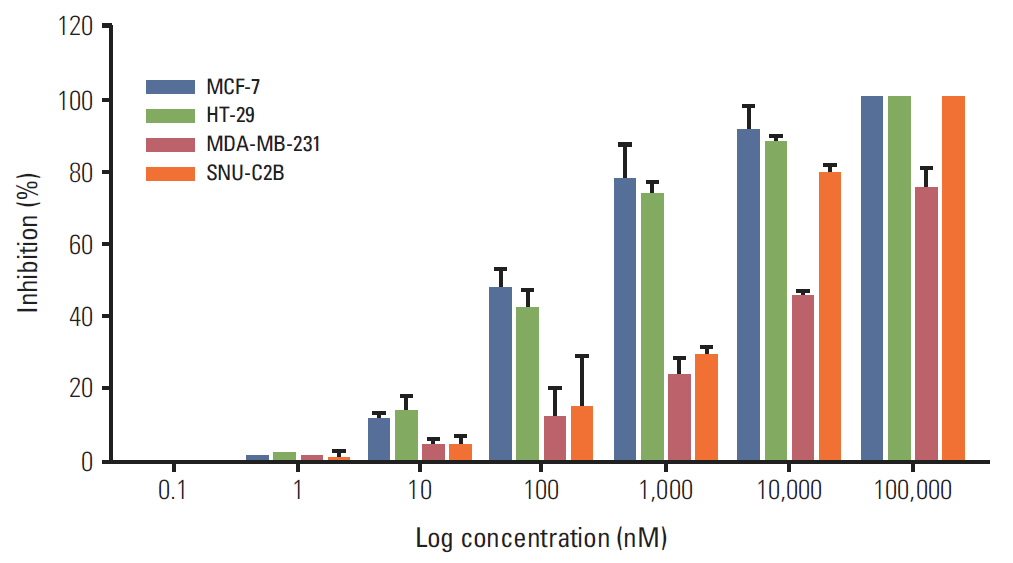
BIA-6 was tested for in vitro Akt1 inhibition using a fluorescence resonance energy transfer kit. Anti-proliferative activity was tested in NCI-H460, A549, NCI-H1975, and NCI-H2170 cell lines. The effect of the compound on p-Akt (S473) was estimated.
RESULTS
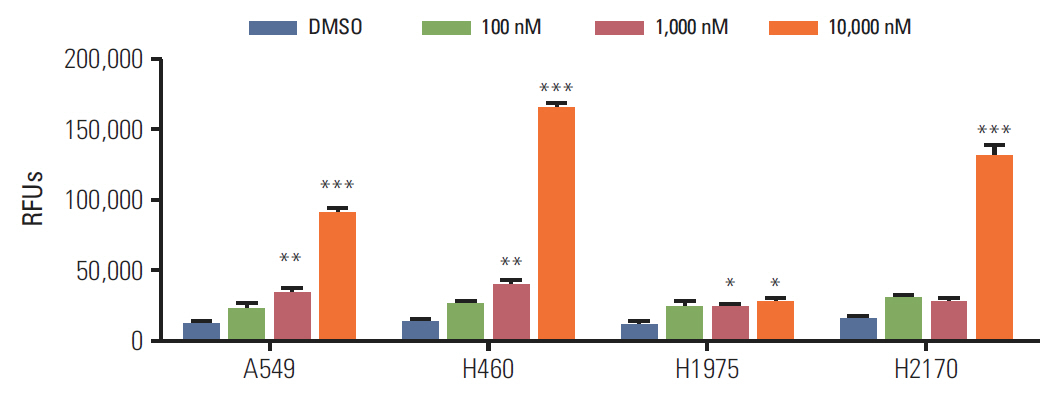
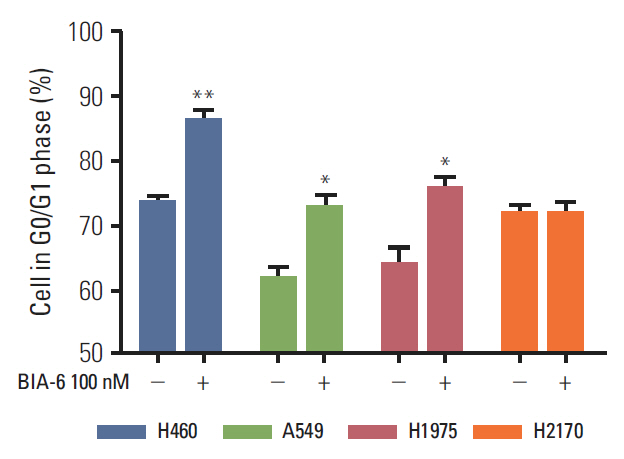
BIA-6 allosterically caused a dose dependent reduction of growth of cell lines with a half maximal growth inhibition (GI50) range of 0.49 muM to 6.6 muM. Cell cycle analysis indicated that BIA-6 caused a G1 phase arrest at < 100 nM but led to apoptosis at higher doses. BIA-6 also exhibited synergism with standard chemotherapeutic agents.
CONCLUSION
BIA-6 is a novel, allosteric Akt inhibitor with potent anti-cancer activity in lung cancer cell lines, that effectively blocks the phosphoinositide-3 kinase/Akt pathway with a high margin selectivity towards normal cells.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–917.
Article2. Shepherd FA, Carney DN. Treatment of NSCLC: chemotherapy. In : Heine HH, editor. Textbook of lung cancer. London: Martin Dunitz Ltd.;2000. p. 213–42.3. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006; 355:2542–50.
Article4. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009; 27:1227–34.
Article5. Zhu J, Sharma DB, Gray SW, Chen AB, Weeks JC, Schrag D. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA. 2012; 307:1593–601.
Article6. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005; 353:123–32.
Article7. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.
Article8. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–46.9. Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999; 96:4240–5.
Article10. Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997; 387:673–6.
Article11. Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997; 7:261–9.12. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007; 129:1261–74.
Article13. Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010; 28:1075–83.
Article14. Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009; 8:2502–8.
Article15. Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005; 4:988–1004.
Article16. Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, et al. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005; 15:761–4.
Article17. Barnett SF, Defeo-Jones D, Fu S, Hancock PJ, Haskell KM, Jones RE, et al. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem J. 2005; 385(Pt 2):399–408.
Article18. Pal SK, Reckamp K, Yu H, Figlin RA. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs. 2010; 19:1355–66.
Article19. Dinavahi SS, Alokam R, Viswanadha S, Dharmarajan S, Permual Y. BIA-6: a novel Akt inhibitor with potent activity in lung cancer. J Thorac Oncol. 2013; 8(Suppl 2):S567.20. Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010; 70:440–6.
Article21. Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002; 2:489–501.22. Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007; 12:487–502.
Article23. Azzoli CG, Baker S Jr, Temin S, Pao W, Aliff T, Brahmer J, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009; 27:6251–66.
Article24. Giovannetti E, Mey V, Nannizzi S, Pasqualetti G, Marini L, Del Tacca M, et al. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol. 2005; 68:110–8.
Article25. Clegg A, Scott DA, Hewitson P, Sidhu M, Waugh N. Clinical and cost effectiveness of paclitaxel, docetaxel, gemcitabine, and vinorelbine in non-small cell lung cancer: a systematic review. Thorax. 2002; 57:20–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dasatinib induces apoptosis and autophagy by suppressing the PI3K/Akt/mTOR pathway in bladder cancer cells
- cAMP signaling increases histone deacetylase 8 expression via the Epac2–Rap1A–Akt pathway in H1299 lung cancer cells
- The Relationship between the serum intercellular adhesion molecule-1 level and the prognosis of the disease in lung cancer
- Small Molecule Inhibitors to Disrupt Protein-protein Interactions of Heat Shock Protein 90 Chaperone Machinery
- Inhibition of STAT3 signaling induces apoptosis and suppresses growth of lung cancer: good and bad