J Vet Sci.
2018 Jan;19(1):79-87. 10.4142/jvs.2018.19.1.79.
Innate immune response of bovine mammary epithelial cells to Mycoplasma bovis
- Affiliations
-
- 1Animal Health Laboratory, Graduate School of Veterinary Medicine, Rakuno Gakuen University, Ebetsu 069-8501, Japan. higuchi@rakuno.ac.jp
- 2Department of Veterinary Biochemistry, Graduate School of Veterinary Medicine, Rakuno Gakuen University, Ebetsu 069-8501, Japan.
- 3National Agricultural Research Center for Hokkaido Region, National Agriculture and Food Research Organization, Sapporo 062-8555, Japan.
- KMID: 2402698
- DOI: http://doi.org/10.4142/jvs.2018.19.1.79
Abstract
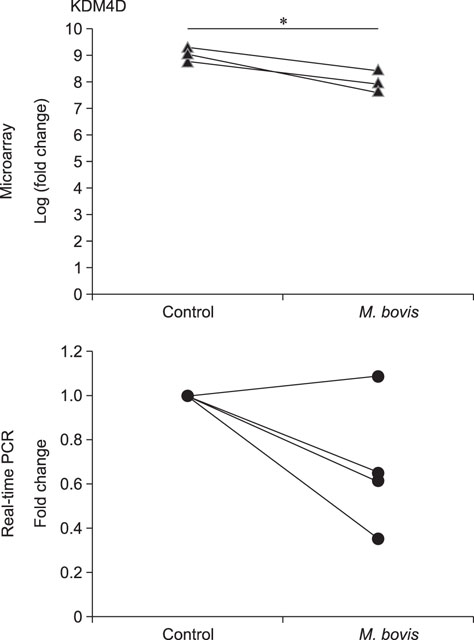
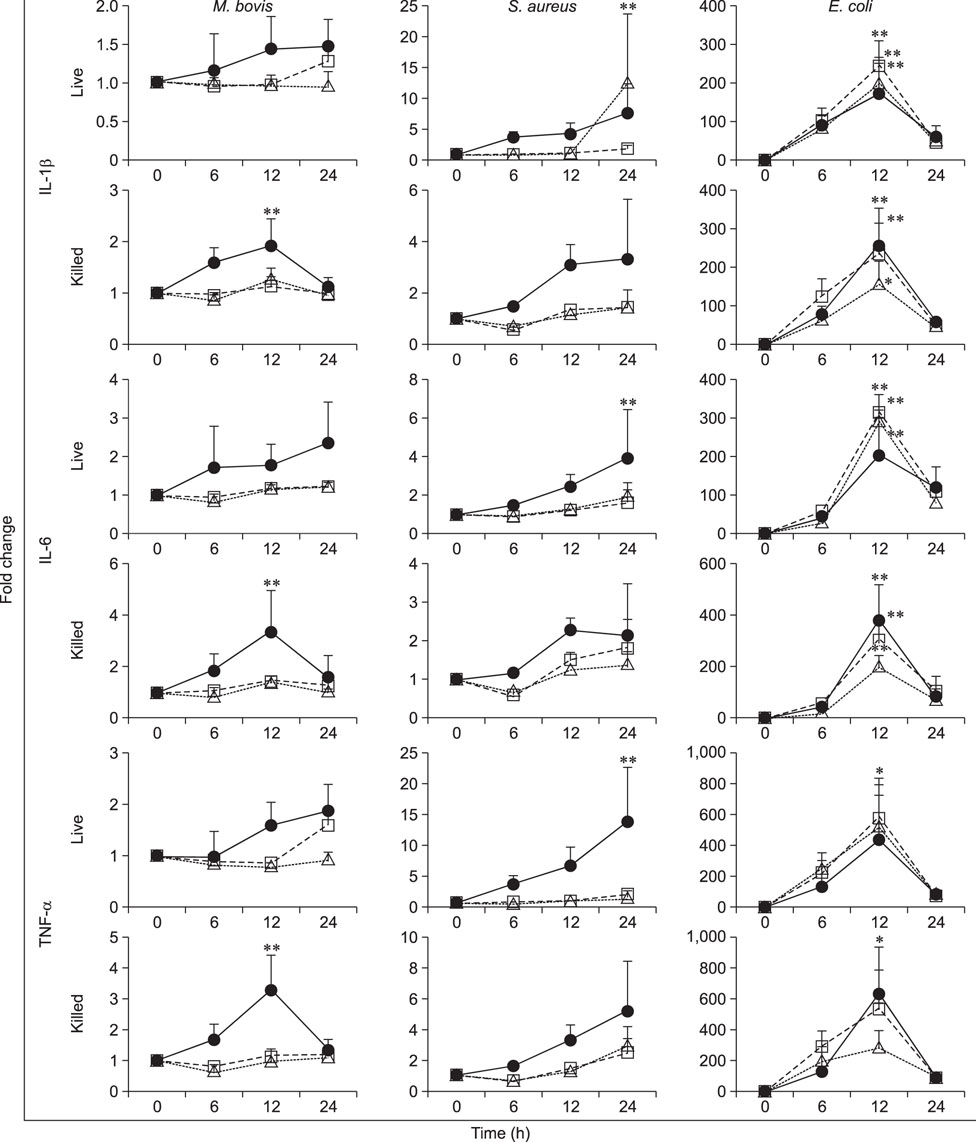
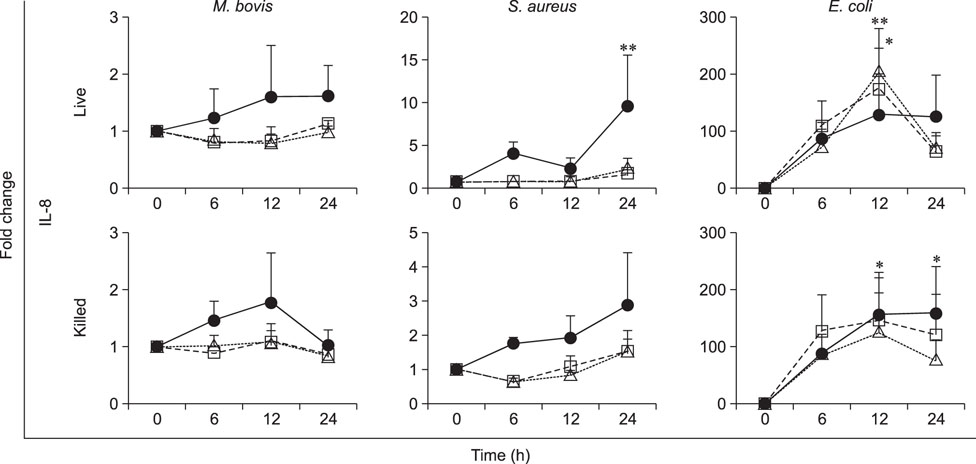
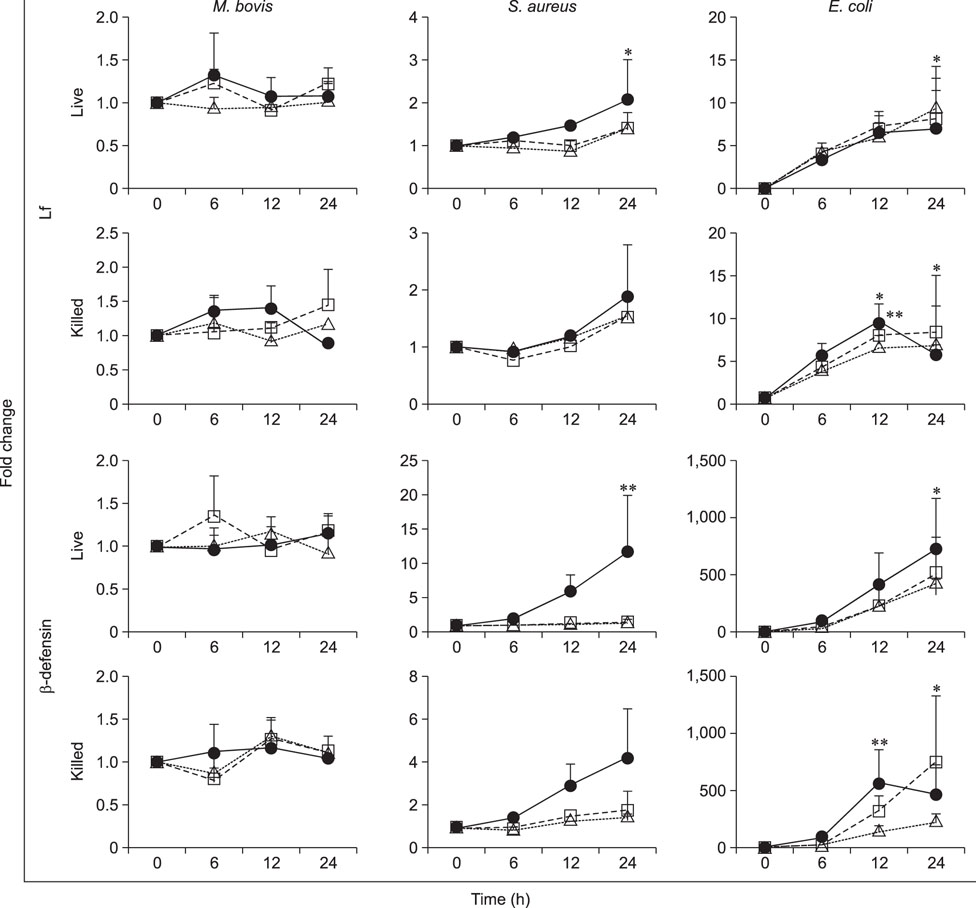
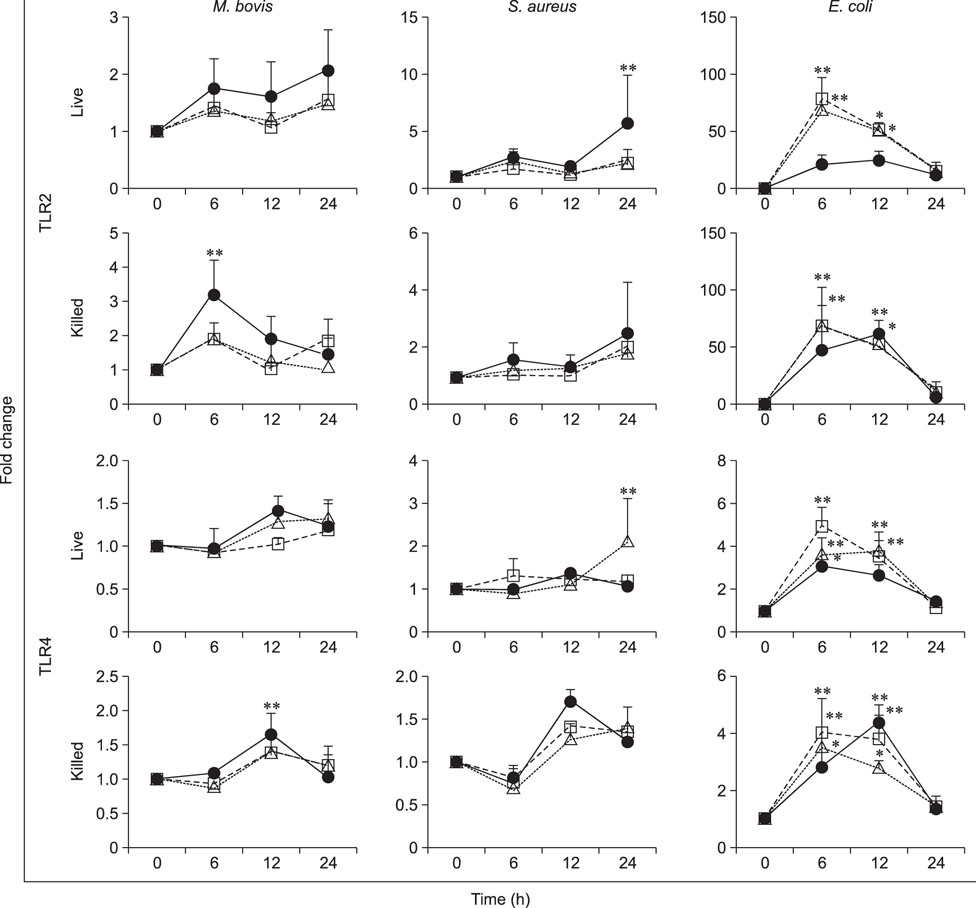
- Mycoplasma spp. are contagious bacteria, and mycoplasmal mastitis is a serious productivity problem on dairy farms. Bovine mammary epithelial cells (bMECs) have an important role in the elimination of pathogens, but the effect of Mycoplasma bovis on bMECs has not been fully described. To elucidate the immune response against intramammary infection by M. bovis, we undertook microarray analysis to examine and profile mRNA expression in bMECs after stimulation with M. bovis. We also compared the effects of M. bovis, Staphylococcus aureus, and Escherichia coli on immune-related mRNA expression in bMECs. Transcriptome analysis indicated a significant decrease in the level of mRNA-encoding lysine-specific demethylase 4D, suggesting that the immune response is suppressed by a decrease in histone demethylase activity. Interleukin (IL)-1β, IL-6, tumor necrosis factor alpha, toll-like receptor (TLR) 2, and TLR4 mRNA expression levels were significantly increased in bMECs stimulated with heat-killed M. bovis, but the expression levels were lower than those following stimulation by heat-killed S. aureus or E. coli. Our results suggest that M. bovis weakly affects mRNA expression in bMECs compared to the effects of E. coli or S. aureus. Moreover, live M. bovis may induce suppression of the immune response in bMECs.
Keyword
MeSH Terms
-
Agriculture
Bacteria
Cytokines
Efficiency
Epithelial Cells*
Escherichia coli
Female
Gene Expression Profiling
Histones
Immunity, Innate*
Interleukin-6
Interleukins
Mastitis
Microarray Analysis
Mycoplasma bovis*
Mycoplasma*
RNA, Messenger
Staphylococcus aureus
Toll-Like Receptors
Tumor Necrosis Factor-alpha
Cytokines
Histones
Interleukin-6
Interleukins
RNA, Messenger
Toll-Like Receptors
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Boulanger D, Bureau F, Mélotte D, Mainil J, Lekeux P. Increased nuclear factor kappaB activity in milk cells of mastitis-affected cows. J Dairy Sci. 2003; 86:1259–1267.
Article2. Brand B, Hartmann A, Repsilber D, Griesbeck-Zilch B, Wellnitz O, Kühn C, Ponsuksili S, Meyer HH, Schwerin M. Comparative expression profiling of E. coli and S. aureus inoculated primary mammary gland cells sampled from cows with different genetic predispositions for somatic cell score. Genet Sel Evol. 2011; 43:24.3. Bürki S, Frey J, Pilo P. Virulence, persistence and dissemination of Mycoplasma bovis. Vet Microbiol. 2015; 179:15–22.4. Chain B, Bowen H, Hammond J, Posch W, Rasaiyaah J, Tsang J, Noursadeghi M. Error, reproducibility and sensitivity: a pipeline for data processing of Agilent oligonucleotide expression arrays. BMC Bioinformatics. 2010; 11:344.
Article5. Fox LK. Mycoplasma mastitis: causes, transmission, and control. Vet Clin North Am Food Anim Pract. 2012; 28:225–237.6. Fu Y, Zhou E, Liu Z, Li F, Liang D, Liu B, Song X, Zhao F, Fen X, Li D, Cao Y, Zhang X, Zhang N, Yang Z. Staphylococcus aureus and Escherichia coli elicit different innate immune responses from bovine mammary epithelial cells. Vet Immunol Immunopathol. 2013; 155:245–252.
Article7. Gilbert FB, Cunha P, Jensen K, Glass EJ, Foucras G, Robert-Granié C, Rupp R, Rainard P. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet Res. 2013; 44:40.8. Gondaira S, Higuchi H, Iwano H, Nakajima K, Kawai K, Hashiguchi S, Konnai S, Nagahata H. Cytokine mRNA profiling and the proliferative response of bovine peripheral blood mononuclear cells to Mycoplasma bovis. Vet Immunol Immunopathol. 2015; 165:45–53.
Article9. Griesbeck-Zilch B, Meyer HH, Kühn CH, Schwerin M, Wellnitz O. Staphylococcus aureus and Escherichia coli cause deviating expression profiles of cytokines and lactoferrin messenger ribonucleic acid in mammary epithelial cells. J Dairy Sci. 2008; 91:2215–2224.
Article10. Günther J, Esch K, Poschadel N, Petzl W, Zerbe H, Mitterhuemer S, Blum H, Seyfert HM. Comparative kinetics of Escherichia coli- and Staphylococcus aureus-specific activation of key immune pathways in mammary epithelial cells demonstrates that S. aureus elicits a delayed response dominated by interleukin-6 (IL-6) but not by IL-1A or tumor necrosis factor alpha. Infect Immun. 2011; 79:695–707.
Article11. Kauf AC, Rosenbusch RF, Paape MJ, Bannerman DD. Innate immune response to intramammary Mycoplasma bovis infection. J Dairy Sci. 2007; 90:3336–3348.12. Krishnan S, Trievel RC. Structural and functional analysis of JMJD2D reveals molecular basis for site-specific demethylation among JMJD2 demethylases. Structure. 2013; 21:98–108.
Article13. Lahouassa H, Moussay E, Rainard P, Riollet C. Differential cytokine and chemokine responses of bovine mammary epithelial cells to Staphylococcus aureus and Escherichia coli. Cytokine. 2007; 38:12–21.
Article14. Maunsell FP, Woolums AR, Francoz D, Rosenbusch RF, Step DL, Wilson DJ, Janzen ED. Mycoplasma bovis infections in cattle. J Vet Intern Med. 2011; 25:772–783.15. Nakajima K, Nakamura M, Gao XD, Kozakai T. Possible involvement of prolactin in the synthesis of lactoferrin in bovine mammary epithelial cells. Biosci Biotechnol Biochem. 2008; 72:1103–1106.
Article16. Nicholas RA. Bovine mycoplasmosis: silent and deadly. Vet Rec. 2011; 168:459–462.
Article17. Nicholas RA, Ayling RD. Mycoplasma bovis: disease, diagnosis, and control. Res Vet Sci. 2003; 74:105–112.18. Rainard P, Riollet C. Innate immunity of the bovine mammary gland. Vet Res. 2006; 37:369–400.
Article19. Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998; 62:1094–1156.
Article20. Robinson TL, Sutherland IA, Sutherland J. Validation of candidate bovine reference genes for use with real-time PCR. Vet Immunol Immunopathol. 2007; 115:160–165.
Article21. Selsted ME, Tang YQ, Morris WL, McGuire PA, Novotny MJ, Smith W, Henschen AH, Cullor JS. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem. 1993; 268:6641–6648.
Article22. Shimizu T. Inflammation-inducing factors of Mycoplasma pneumoniae. Front Microbiol. 2016; 7:414.23. Shio MT, Hassan GS, Shah WA, Nadiri A, El Fakhry Y, Li H, Mourad W. Coexpression of TLR2 or TLR4 with HLA-DR potentiates the superantigenic activities of Mycoplasma arthritidis-derived mitogen. J Immunol. 2014; 192:2543–2550.
Article24. Spalenza V, Girolami F, Bevilacqua C, Riondato F, Rasero R, Nebbia C, Sacchi P, Martin P. Identification of internal control genes for quantitative expression analysis by real-time PCR in bovine peripheral lymphocytes. Vet J. 2011; 189:278–283.
Article25. Ulvatne H, Vorland LH. Bactericidal kinetics of 3 lactoferricins against Staphylococcus aureus and Escherichia coli. Scand J Infect Dis. 2001; 33:507–511.26. Zhu Y, van Essen D, Saccani S. Cell-type-specific control of enhancer activity by H3K9 trimethylation. Mol Cell. 2012; 46:408–423.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Innate Immune Responses in Pathogenesis of Chronic Rhinosinusitis
- Recent advance in primary immune deficiency disorders
- Innate immune response to oral bacteria and the immune evasive characteristics of periodontal pathogens
- The Role of Type 2 Innate Lymphoid Cells in Allergic Diseases
- Airway epithelial cells in airway inflammation and remodeling in asthma