Asia Pac Allergy.
2014 Jan;4(1):14-18. 10.5415/apallergy.2014.4.1.14.
Epigenetic regulation in allergic diseases and related studies
- Affiliations
-
- 1Department of Pediatrics, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung 80708, Taiwan.
- 2Department of Pediatrics, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 80708, Taiwan. pedhung@gmail.com
- 3Division of Cardiovascular Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung 80708, Taiwan.
- 4Department of Pediatrics, Kaohsiung Municipal Hsiao-Kang Hospital, Kaohsiung 80708, Taiwan.
- 5Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung 80708, Taiwan.
- 6Department of Internal Medicine, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung 80708, Taiwan.
- 7Department of Pediatrics, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80708, Taiwan.
- 8Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80708, Taiwan.
- KMID: 2397120
- DOI: http://doi.org/10.5415/apallergy.2014.4.1.14
Abstract
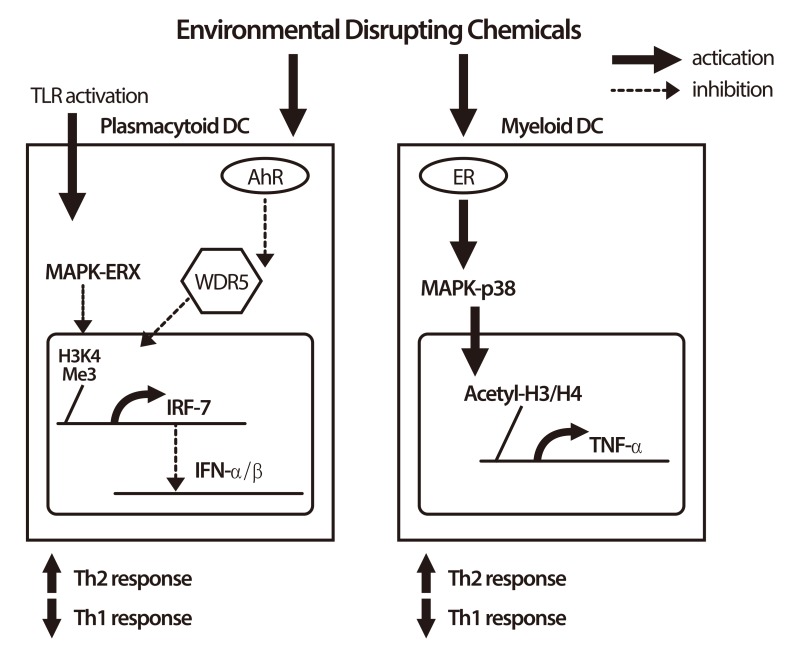
- Asthma, a chronic inflammatory disorder of the airway, has features of both heritability as well as environmental influences which can be introduced in utero exposures and modified through aging, and the features may attribute to epigenetic regulation. Epigenetic regulation explains the association between early prenatal maternal smoking and later asthma-related outcomes. Epigenetic marks (DNA methylation, modifications of histone tails or noncoding RNAs) work with other components of the cellular regulatory machinery to control the levels of expressed genes, and several allergy- and asthma-related genes have been found to be susceptible to epigenetic regulation, including genes important to T-effector pathways (IFN-γ, interleukin [IL] 4, IL-13, IL-17) and T-regulatory pathways (FoxP3). Therefore, the mechanism by which epigenetic regulation contributes to allergic diseases is a critical issue. In the past most published experimental work, with few exceptions, has only comprised small observational studies and models in cell systems and animals. However, very recently exciting and elegant experimental studies and novel translational research works were published with new and advanced technologies investigating epigenetic mark on a genomic scale and comprehensive approaches to data analysis. Interestingly, a potential link between exposure to environmental pollutants and the occurrence of allergic diseases is revealed recently, particular in developed and industrialized countries, and endocrine disrupting chemicals (EDCs) as environmental hormone may play a key role. This review addresses the important question of how EDCs (nonylphenol, 4 octylphenol, and phthalates) influences on asthma-related gene expression via epigenetic regulation in immune cells, and how anti-asthmatic agents prohibit expression of inflammatory genes via epigenetic modification. The discovery and validation of epigenetic biomarkers linking exposure to allergic diseases might lead to better epigenotyping of risk, prognosis, treatment prediction, and development of novel therapies.
Keyword
MeSH Terms
-
Acetylation
Aging
Animals
Anti-Asthmatic Agents
Asthma
Biomarkers
Developed Countries
Endocrine Disruptors
Environmental Pollutants
Epigenomics*
Gene Expression
Histones
Hypersensitivity
Interleukin-13
Interleukins
Methylation
Prognosis
Smoke
Smoking
Statistics as Topic
Tail
Translational Medical Research
Anti-Asthmatic Agents
Biomarkers
Endocrine Disruptors
Environmental Pollutants
Histones
Interleukin-13
Interleukins
Smoke
Figure
Cited by 1 articles
-
Asia Pacific, and beyond
Yoon-Seok Chang, Sang-Il Lee
Asia Pac Allergy. 2014;4(1):1-2. doi: 10.5415/apallergy.2014.4.1.1.
Reference
-
1. Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003; 111:215–225. PMID: 12589337.2. Esteller M. Epigenetics in evolution and disease. Lancet. 2008; 372:S90–S96.
Article3. Schoenborn JR, Dorschner MO, Sekimata M, Santer DM, Shnyreva M, Fitzpatrick DR, Stamatoyannopoulos JA, Wilson CB. Comprehensive epigenetic profiling identifies multiple distal regulatory elements directing transcription of the gene encoding interferon-gamma. Nat Immunol. 2007; 8:732–742. PMID: 17546033.4. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006; 355:2226–2235. PMID: 17124020.
Article5. Lee DU, Agarwal S, Rao A. Th2 lineage commitment and efficient IL-4 production involves extended demethylation of the IL-4 gene. Immunity. 2002; 16:649–660. PMID: 12049717.
Article6. Tykocinski LO, Hajkova P, Chang HD, Stamm T, Sözeri O, Löhning M, Hu-Li J, Niesner U, Kreher S, Friedrich B, Pannetier C, Grutz G, Walter J, Paul WE, Radbruch A. A critical control element for interleukin-4 memory expression in T helper lymphocytes. J Biol Chem. 2005; 280:28177–28185. PMID: 15941711.
Article7. Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, Schones DE, Peng W, Sun HW, Paul WE, O'Shea JJ, Zhao K. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009; 30:155–167. PMID: 19144320.
Article8. Fields PE, Kim ST, Flavell RA. Cutting edge: changes in histone acetylation at the IL-4 and IFN-gamma loci accompany Th1/Th2 differentiation. J Immunol. 2002; 169:647–650. PMID: 12097365.9. White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO-T cells. J Immunol. 2002; 168:2820–2827. PMID: 11884451.10. Jones B, Chen J. Inhibition of IFN-gamma transcription by site-specific methylation during T helper cell development. EMBO J. 2006; 25:2443–2452. PMID: 16724115.11. Schones DE, Zhao K. Genome-wide approaches to studying chromatin modifications. Nat Rev Genet. 2008; 9:179–191. PMID: 18250624.
Article12. Harris RA, Wang T, Coarfa C, Nagarajan RP, Hong C, Downey SL, Johnson BE, Fouse SD, Delaney A, Zhao Y, Olshen A, Ballinger T, Zhou X, Forsberg KJ, Gu J, Echipare L, O'Geen H, Lister R, Pelizzola M, Xi Y, Epstein CB, Bernstein BE, Hawkins RD, Ren B, Chung WY, Gu H, Bock C, Gnirke A, Zhang MQ, Haussler D, Ecker JR, Li W, Farnham PJ, Waterland RA, Meissner A, Marra MA, Hirst M, Milosavljevic A, Costello JF. Comparison of sequencing-based methods to profile DNA methylation and identification of monoallelic epigenetic modifications. Nat Biotechnol. 2010; 28:1097–1105. PMID: 20852635.
Article13. Brinkman AB, Gu H, Bartels SJ, Zhang Y, Matarese F, Simmer F, Marks H, Bock C, Gnirke A, Meissner A, Stunnenberg HG. Sequential ChIP-bisulfite sequencing enables direct genome-scale investigation of chromatin and DNA methylation cross-talk. Genome Res. 2012; 22:1128–1138. PMID: 22466170.
Article14. Statham AL, Robinson MD, Song JZ, Coolen MW, Stirzaker C, Clark SJ. Bisulfite sequencing of chromatin immunoprecipitated DNA (BisChIP-seq) directly informs methylation status of histone-modified DNA. Genome Res. 2012; 22:1120–1127. PMID: 22466171.
Article15. Song CX, Clark TA, Lu XY, Kislyuk A, Dai Q, Turner SW, He C, Korlach J. Sensitive and specific single-molecule sequencing of 5-hydroxymethylcytosine. Nat Methods. 2011; 9:75–77. PMID: 22101853.
Article16. Alati R, Al Mamun A, O'Callaghan M, Najman JM, Williams GM. In utero and postnatal maternal smoking and asthma in adolescence. Epidemiology. 2006; 17:138–144. PMID: 16477253.
Article17. Hung CH, Yang SN, Kuo PL, Chu YT, Chang HW, Wei WJ, Huang SK, Jong YJ. Modulation of cytokine expression in human myeloid dendritic cells by environmental endocrine-disrupting chemicals involves epigenetic regulation. Environ Health Perspect. 2010; 118:67–72. PMID: 20056579.
Article18. Kuo CH, Hsieh CC, Kuo HF, Huang MY, Yang SN, Chen LC, Huang SK, Hung CH. Phthalates suppress type I interferon in human plasmacytoid dendritic cells via epigenetic regulation. Allergy. 2013; 68:870–879. PMID: 23738920.
Article19. Idzko M, Hammad H, van Nimwegen M, Kool M, Vos N, Hoogsteden HC, Lambrecht BN. Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. J Clin Invest. 2007; 117:464–472. PMID: 17273558.
Article20. Hung CH, Chu YT, Suen JL, Lee MS, Chang HW, Lo YC, Jong YJ. Regulation of cytokine expression in human plasmacytoid dendritic cells by prostaglandin I2 analogues. Eur Respir J. 2009; 33:405–410. PMID: 19181914.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epigenetics in Allergic Diseases
- Effects of Maternal Diet during Pregnancy or Lactation on the Development or Prevention of Allergic Diseases in Offspring
- Epigenetic regulation and chromatin remodeling in learning and memory
- Epigenetic Regulation of Cytokine Gene Expression in T Lymphocytes
- Impact of perinatal environmental tobacco smoke on the development of childhood allergic diseases