Int J Thyroidol.
2017 Nov;10(2):71-76. 10.11106/ijt.2017.10.2.71.
Enhancement of Osteogenic Differentiation by Combination Treatment with 5-azacytidine and Thyroid-Stimulating Hormone in Human Osteoblast Cells
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. swchomd@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2396809
- DOI: http://doi.org/10.11106/ijt.2017.10.2.71
Abstract
- BACKGROUND AND OBJECTIVES
The role of thyroid-stimulating hormone (TSH) signaling on osteoblastic differentiation is still undetermined. The aim of this study was to investigate the effects of 5-aza-2"²-deoxycytidine (5-azacytidine) on TSH-mediated regulations of osteoblasts.
MATERIALS AND METHODS
MG63, a human osteoblastic cell-line, was treated with 5-azacytidine before inducing osteogenic differentiation using osteogenic medium (OM) containing L-ascorbic acid and β-glyceophosphate. Bovine TSH or monoclonal TSH receptor stimulating antibody (TSAb) was treated. Quantitative real-time PCR analyses or measurement of alkaline phosphatase activities were performed for evaluating osteoblastic differentiation.
RESULTS
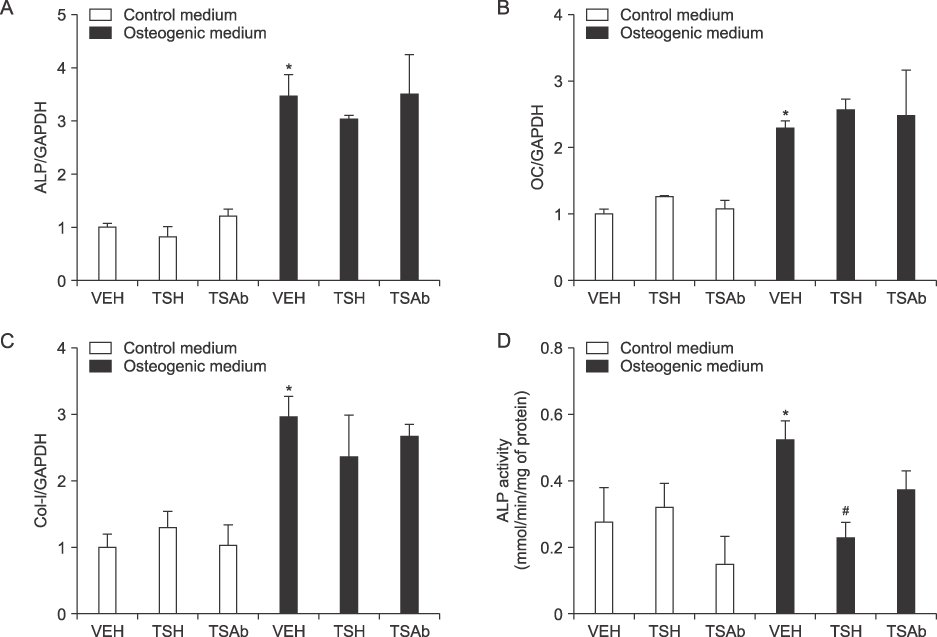
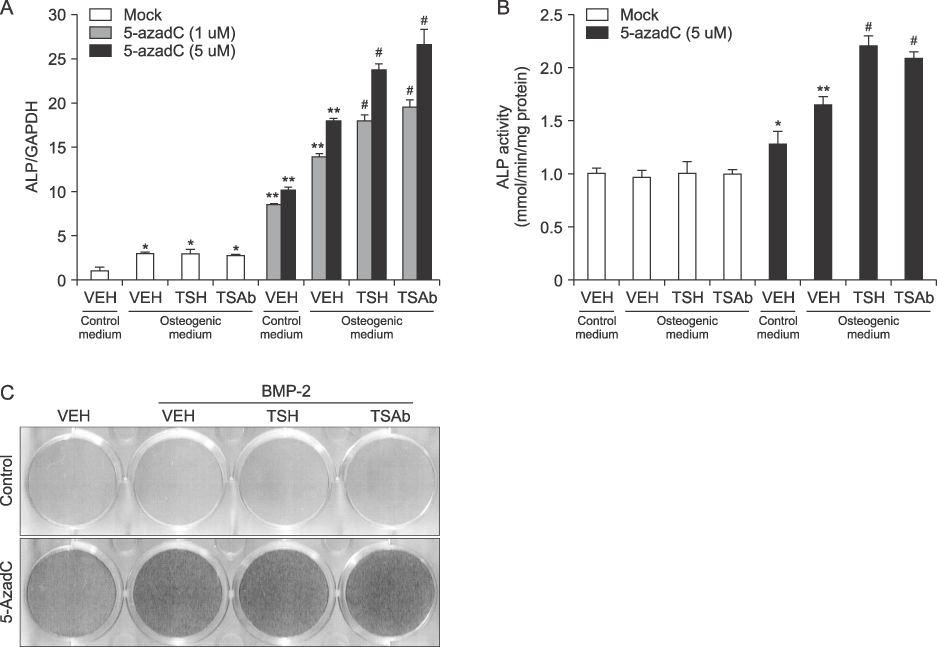
Studies for osteogenic-related genes or alkaline phosphatase activity demonstrated that treatment of TSH or TSAb alone had no effects on osteoblastic differentiation in MG63 cells. However, treatment of 5-azacytidine, per se, significantly increased osteoblastic differentiation and combination treatment of 5-azacytidine and TSH or TSAb in the condition of OM showed further significant increase of osteoblastic differentiation.
CONCLUSION
Stimulating TSH signaling has little effects on osteoblastic differentiation in vitro. However, in the condition of epigenetic modification using inhibitor of DNA methylation, TSH signaling positively affects osteoblastic differentiation in human osteoblasts.
MeSH Terms
Figure
Reference
-
1. Bassett JH, Williams GR. The molecular actions of thyroid hormone in bone. Trends Endocrinol Metab. 2003; 14(8):356–364.
Article2. Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, da Costa BR, et al. Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA. 2015; 313(20):2055–2065.3. Murphy E, Gluer CC, Reid DM, Felsenberg D, Roux C, Eastell R, et al. Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. J Clin Endocrinol Metab. 2010; 95(7):3173–3181.
Article4. Sun L, Davies TF, Blair HC, Abe E, Zaidi M. TSH and bone loss. Ann N Y Acad Sci. 2006; 1068:309–318.
Article5. Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003; 115(2):151–162.
Article6. Tuncel M, Aydin D, Yaman E, Tazebay UH, Guc D, Dogan AL, et al. The comparative effects of gene modulators on thyroid-specific genes and radioiodine uptake. Cancer Biother Radiopharm. 2007; 22(3):443–449.
Article7. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008; 3(6):1101–1108.
Article8. Baran DT. Thyroid hormone and bone mass: the clinician's dilemma. Thyroid. 1994; 4(2):143–144.
Article9. Liu XS, Sajda P, Saha PK, Wehrli FW, Guo XE. Quantification of the roles of trabecular microarchitecture and trabecular type in determining the elastic modulus of human trabecular bone. J Bone Miner Res. 2006; 21(10):1608–1617.
Article10. Hase H, Ando T, Eldeiry L, Brebene A, Peng Y, Liu L, et al. TNFalpha mediates the skeletal effects of thyroid-stimulating hormone. Proc Natl Acad Sci U S A. 2006; 103(34):12849–12854.11. Sun L, Zhu LL, Lu P, Yuen T, Li J, Ma R, et al. Genetic confirmation for a central role for TNFalpha in the direct action of thyroid stimulating hormone on the skeleton. Proc Natl Acad Sci U S A. 2013; 110(24):9891–9896.
Article12. Baliram R, Latif R, Berkowitz J, Frid S, Colaianni G, Sun L, et al. Thyroid-stimulating hormone induces a Wnt-dependent, feed-forward loop for osteoblastogenesis in embryonic stem cell cultures. Proc Natl Acad Sci U S A. 2011; 108(39):16277–16282.
Article13. Sampath TK, Simic P, Sendak R, Draca N, Bowe AE, O'Brien S, et al. Thyroid-stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. J Bone Miner Res. 2007; 22(6):849–859.
Article14. Sun L, Vukicevic S, Baliram R, Yang G, Sendak R, McPherson J, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci U S A. 2008; 105(11):4289–4294.
Article15. Cho SW, Bae JH, Noh GW, Kim YA, Moon MK, Park KU, et al. The presence of thyroid-stimulation blocking antibody prevents high bone turnover in untreated premenopausal patients with Graves' disease. PLoS One. 2015; 10(12):e0144599.
Article16. de Andres MC, Kingham E, Imagawa K, Gonzalez A, Roach HI, Wilson DI, et al. Epigenetic regulation during fetal femur development: DNA methylation matters. PLoS One. 2013; 8(1):e54957.
Article17. Villagra A, Gutierrez J, Paredes R, Sierra J, Puchi M, Imschenetzky M, et al. Reduced CpG methylation is associated with transcriptional activation of the bone-specific rat osteocalcin gene in osteoblasts. J Cell Biochem. 2002; 85(1):112–122.
Article18. Lee JY, Lee YM, Kim MJ, Choi JY, Park EK, Kim SY, et al. Methylation of the mouse DIx5 and Osx gene promoters regulates cell type-specific gene expression. Mol Cells. 2006; 22(2):182–188.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Macrophage-Stimulating Protein Enhances Osteoblastic Differentiation via the Recepteur d'Origine Nantais Receptor and Extracellular Signal-Regulated Kinase Signaling Pathway
- Effects of Thyroid Stimulating Hormone on Bone Metabolism
- Difference of Thyroid Stimulating Antidody Activities Measured in Chinese Hamster Ovary Cells Stably Transfected with Human TSH Receptor and in FRTL-5 Cells in Graves Disease and Its Clinical Correlations
- Synergistic Effects of Chios Gum Mastic Extract and Low Level Laser Therapy on Osteoblast Differentiation
- Modulation of Osteogenic Differentiation of Adipose-Derived Stromal Cells by Co-Treatment with 3, 4’-Dihydroxyflavone, U0126, and N-Acetyl Cysteine