Clin Exp Otorhinolaryngol.
2017 Dec;10(4):325-331. 10.21053/ceo.2017.00101.
Effect of Proparacaine in a Mouse Model of Allergic Rhinitis
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea. hhkped@catholic.ac.kr
- KMID: 2396787
- DOI: http://doi.org/10.21053/ceo.2017.00101
Abstract
OBJECTIVES
Lidocaine, a local anaesthetic is a treatment option in uncontrolled asthma due to its immunomodulatory effects. In the present study, proparacaine (PPC), a derivative of lidocaine was examined for its therapeutic application in a mouse model of allergic rhinitis.
METHODS
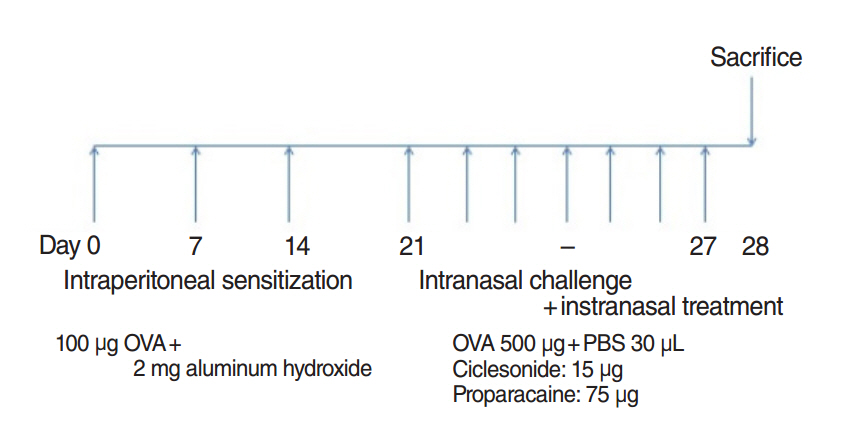
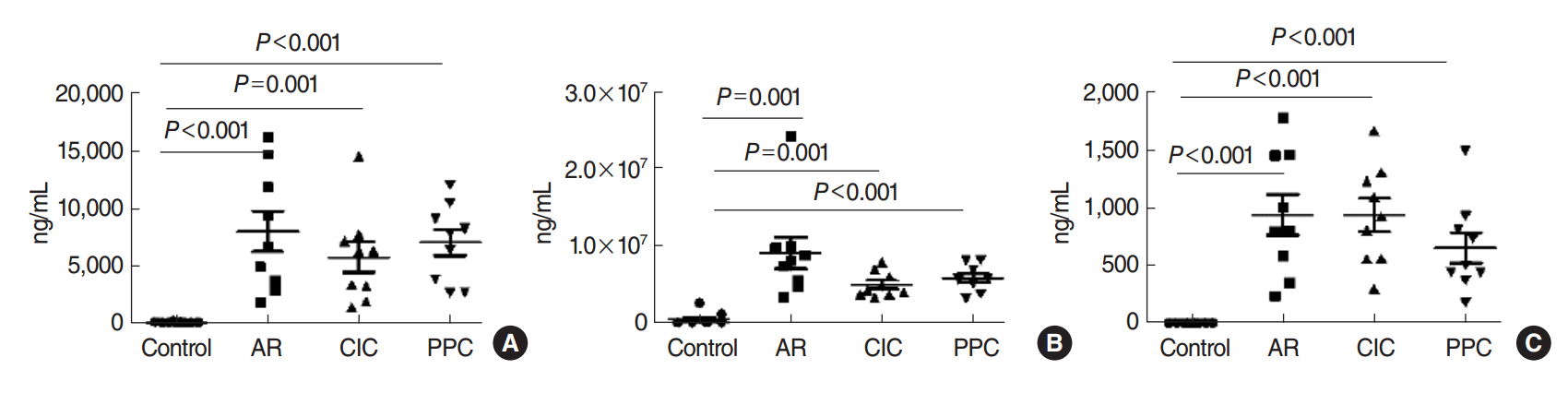
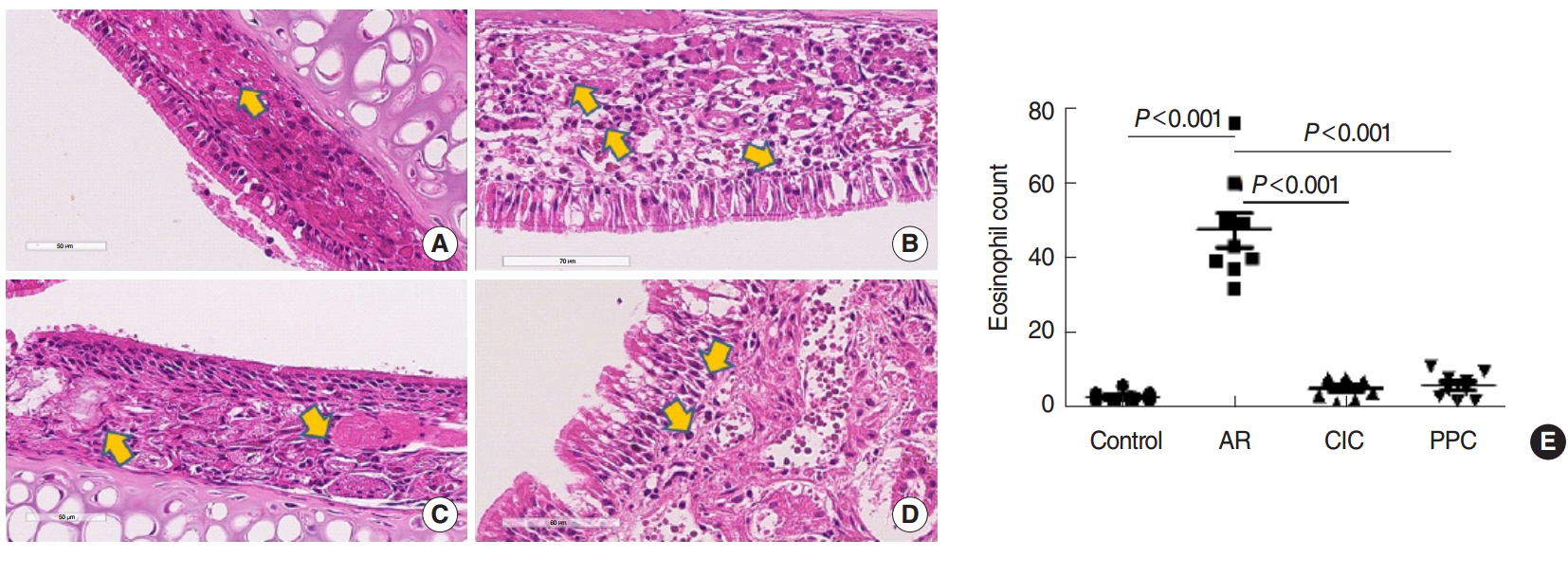
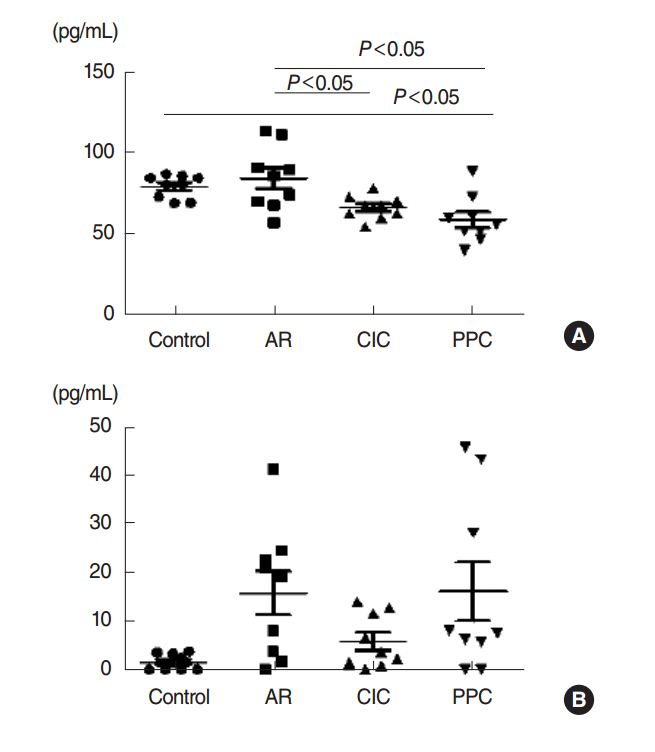
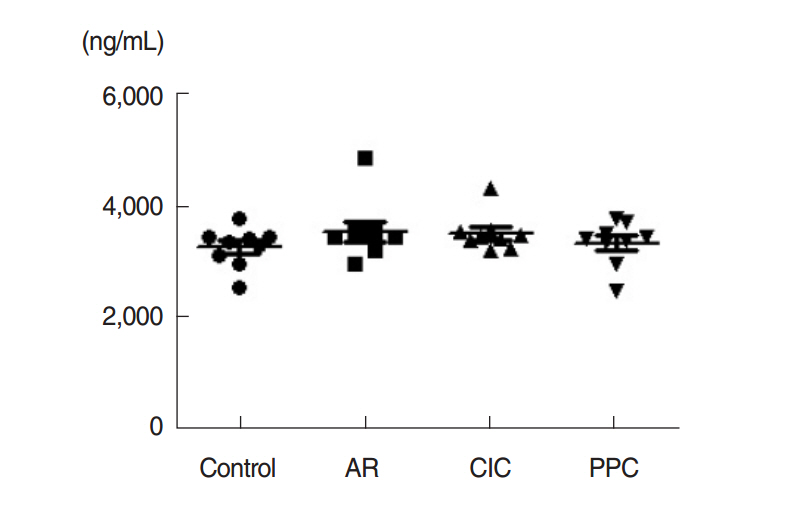
The mice were grouped into 4 groups: control group, allergic rhinitis (AR) group, ciclesonide (CIC) group, and PPC group. Nasal symptom scores, eosinophil counts, goblet cell counts, and mast cells counts in the nasal mucosa were measured. Serum ovalbumin (OVA)-specific immunoglobulin (Ig) E, OVA-specific IgG1, OVA-specific IgG2a, interleukin (IL)-4, IL-5, and cortisol levels were measured.
RESULTS
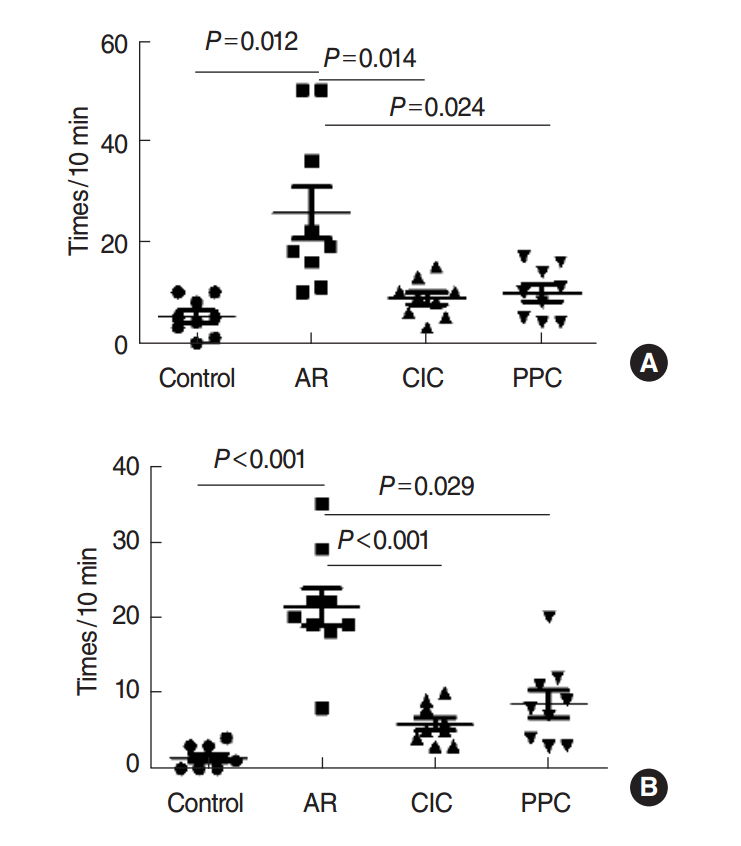
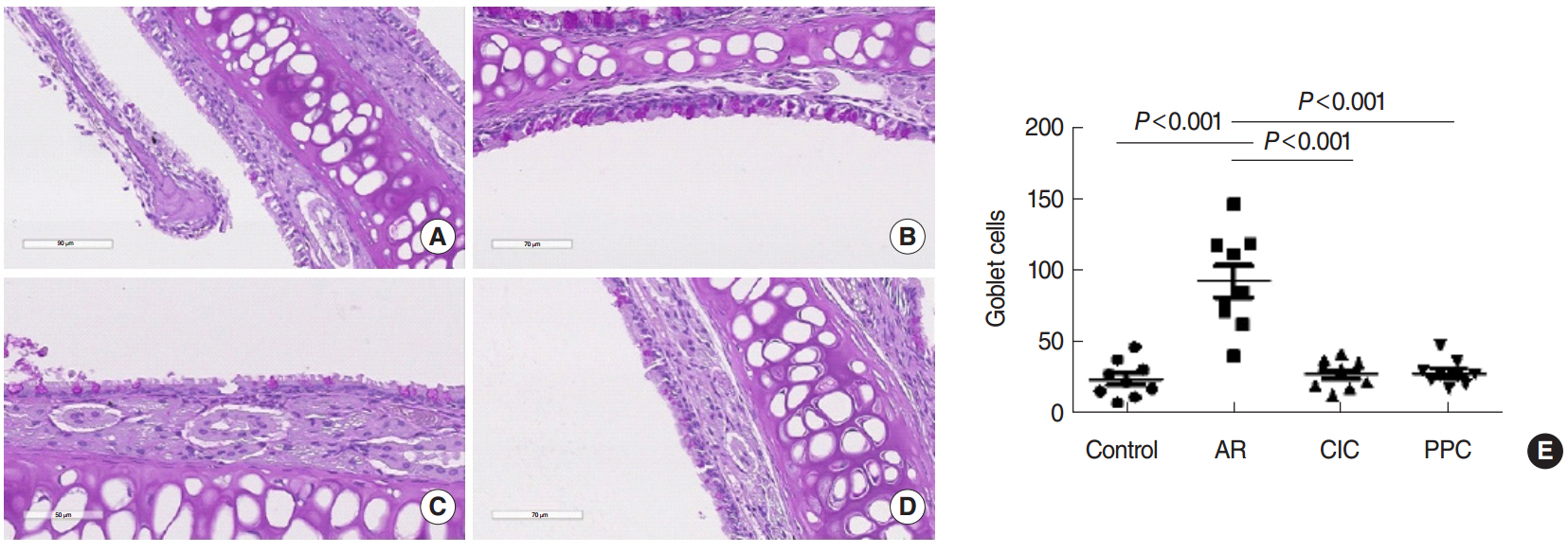
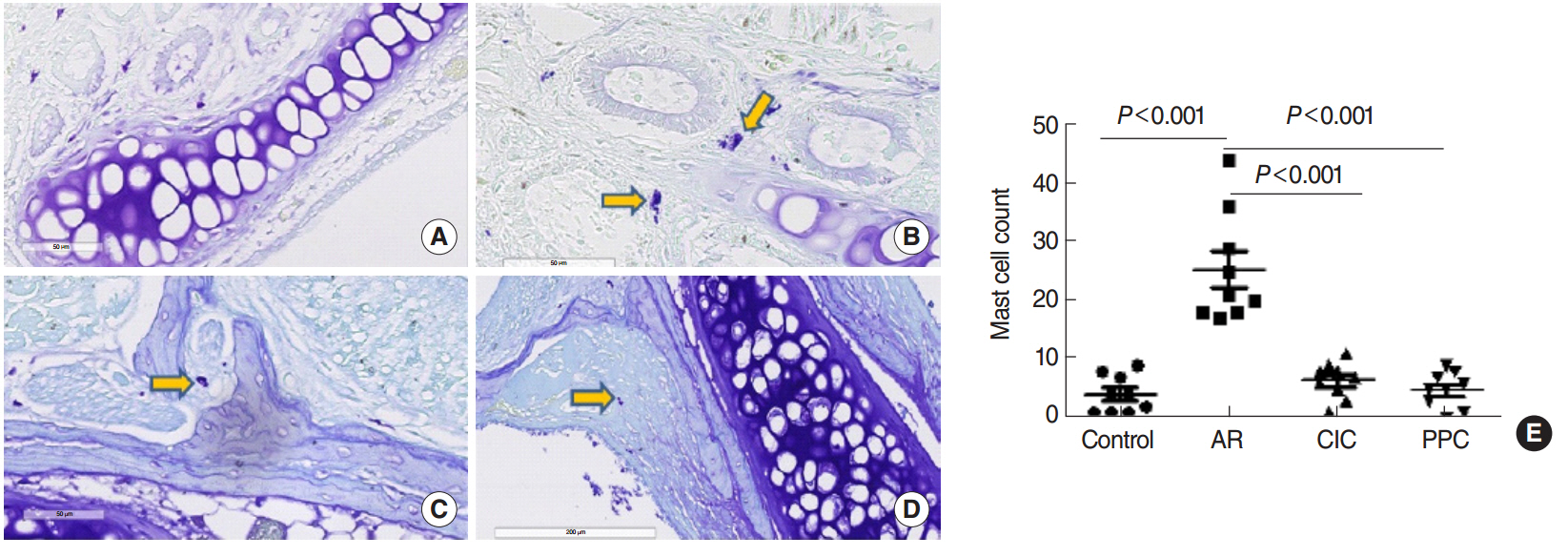
Intranasal administration of PPC significantly decreased nasal symptoms, number of eosinophils, goblet cells, and mast cells in the lamina propria of the nasal mucosa. Serum OVA-specific IgE, OVA-specific IgG1, OVA-specific IgG2a was significantly higher in the AR compared with the control group. Serum level of IL-4 was significantly lower in the CIC group and PPC group in comparison with AR group. Serum IL-5 showed no significant difference among all groups. No significant difference in serum cortisol levels was observed among the 4 groups.
CONCLUSION
PPC appears to have a therapeutic potential in treatment of allergic rhinitis in a mouse model by reducing eosinophil, goblet cell, and mast cell infiltration in the nasal mucosa.
Keyword
MeSH Terms
-
Administration, Intranasal
Animals
Asthma
Eosinophils
Goblet Cells
Hydrocortisone
Immunoglobulin E
Immunoglobulin G
Immunoglobulins
Interleukin-4
Interleukin-5
Interleukins
Lidocaine
Mast Cells
Mice*
Mucous Membrane
Nasal Mucosa
Ovalbumin
Rhinitis, Allergic*
Hydrocortisone
Immunoglobulin E
Immunoglobulin G
Immunoglobulins
Interleukin-4
Interleukin-5
Interleukins
Lidocaine
Ovalbumin
Figure
Reference
-
1. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; Apr. 63 Suppl 86:8–160.2. Nicolaizik WH, Marchant JL, Preece MA, Warner JO. Endocrine and lung function in asthmatic children on inhaled corticosteroids. Am J Respir Crit Care Med. 1994; Sep. 150(3):624–8.
Article3. Phillip M, Aviram M, Leiberman E, Zadik Z, Giat Y, Levy J, et al. Integrated plasma cortisol concentration in children with asthma receiving long-term inhaled corticosteroids. Pediatr Pulmonol. 1992; Feb. 12(2):84–9.
Article4. Toogood JH, Jennings B, Hodsman AB, Baskerville J, Fraher LJ. Effects of dose and dosing schedule of inhaled budesonide on bone turnover. J Allergy Clin Immunol. 1991; Oct. 88(4):572–80.
Article5. Okada S, Hagan JB, Kato M, Bankers-Fulbright JL, Hunt LW, Gleich GJ, et al. Lidocaine and its analogues inhibit IL-5-mediated survival and activation of human eosinophils. J Immunol. 1998; Apr. 160(8):4010–7.6. Serra MF, Anjos-Valotta EA, Olsen PC, Couto GC, Jurgilas PB, Cotias AC, et al. Nebulized lidocaine prevents airway inflammation, peribronchial fibrosis, and mucus production in a murine model of asthma. Anesthesiology. 2012; Sep. 117(3):580–91.
Article7. Goldstein IM, Lind S, Hoffstein S, Weissmann G. Influence of local anesthetics upon human polymorphonuclear leukocyte function in vitro: reduction of lysosomal enzyme release and superoxide anion production. J Exp Med. 1977; Aug. 146(2):483–94.
Article8. Sinclair R, Eriksson AS, Gretzer C, Cassuto J, Thomsen P. Inhibitory effects of amide local anaesthetics on stimulus-induced human leukocyte metabolic activation, LTB4 release and IL-1 secretion in vitro. Acta Anaesthesiol Scand. 1993; Feb. 37(2):159–65.9. Tanaka A, Minoguchi K, Oda N, Yokoe T, Matsuo H, Okada S, et al. Inhibitory effect of lidocaine on T cells from patients with allergic asthma. J Allergy Clin Immunol. 2002; Mar. 109(3):485–90.
Article10. Suzuki T, Ohishi K, Kida J, Uchida M. Influence of pH on the inhibitory effects of local anesthetics on histamine release induced from rat mast cells by concanavalin A and compound 48/80. Eur J Pharmacol. 1984; Mar. 98(3-4):347–55.11. Ohnishi T, Kita H, Mayeno AN, Okada S, Sur S, Broide DH, et al. Lidocaine in bronchoalveolar lavage fluid (BALF) is an inhibitor of eosinophil-active cytokines. Clin Exp Immunol. 1996; May. 104(2):325–31.
Article12. Decco ML, Neeno TA, Hunt LW, O’Connell EJ, Yunginger JW, Sachs MI. Nebulized lidocaine in the treatment of severe asthma in children: a pilot study. Ann Allergy Asthma Immunol. 1999; Jan. 82(1):29–32.
Article13. Lee JE, Zhang YL, Han DH, Kim DY, Rhee CS. Antiallergic function of KR62980, a peroxisome proliferator-activated receptor-γ agonist, in a mouse allergic rhinitis model. Allergy Asthma Immunol Res. 2015; May. 7(3):256–64.
Article14. Hunt LW, Frigas E, Butterfield JH, Kita H, Blomgren J, Dunnette SL, et al. Treatment of asthma with nebulized lidocaine: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2004; May. 113(5):853–9.
Article15. Hunt LW, Swedlund HA, Gleich GJ. Effect of nebulized lidocaine on severe glucocorticoid-dependent asthma. Mayo Clin Proc. 1996; Apr. 71(4):361–8.
Article16. Rosario NA, Riedi CA, Farias L. Lidocaine nebulization for treatment of asthma. Ann Allergy Asthma Immunol. 2000; Sep. 85(3):245–6.
Article17. Talbot S, Abdulnour RE, Burkett PR, Lee S, Cronin SJ, Pascal MA, et al. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron. 2015; Jul. 87(2):341–54.
Article18. Serra MF, Neves JS, Couto GC, Cotias AC, Pao CR, Olsen PC, et al. JM25-1, a lidocaine analog combining airway relaxant and antiinflammatory properties: implications for new bronchospasm therapy. Anesthesiology. 2016; Jan. 124(1):109–20.19. Kessler TL, Mercer HJ, Zieske JD, McCarthy DM, Dartt DA. Stimulation of goblet cell mucous secretion by activation of nerves in rat conjunctiva. Curr Eye Res. 1995; Nov. 14(11):985–92.
Article20. Rodrigues FV, Hochman B, Wood VT, Simoes MJ, Juliano Y, Ferreira LM. Effects of lidocaine with epinephrine or with buffer on wound healing in rat skin. Wound Repair Regen. 2011; Mar-Apr. 19(2):223–8.
Article21. Olsen PC, Ferreira TP, Serra MF, Farias-Filho FA, Fonseca BP, Viola JP, et al. Lidocaine-derivative JMF2-1 prevents ovalbumin-induced airway inflammation by regulating the function and survival of T cells. Clin Exp Allergy. 2011; Feb. 41(2):250–9.
Article22. Church MK, Levi-Schaffer F. The human mast cell. J Allergy Clin Immunol. 1997; Feb. 99(2):155–60.
Article23. Son HL, Park HR, Park YJ, Kim SW. Effect of retinoic acid in a mouse model of allergic rhinitis. Allergy Asthma Immunol Res. 2015; Nov. 7(6):590–8.
Article24. Liang MJ, Fu QL, Jiang HY, Chen FH, Chen D, Chen DH, et al. Immune responses to different patterns of exposure to ovalbumin in a mouse model of allergic rhinitis. Eur Arch Otorhinolaryngol. 2016; Nov. 273(11):3783–8.
Article25. Newton R, Leigh R, Giembycz MA. Pharmacological strategies for improving the efficacy and therapeutic ratio of glucocorticoids in inflammatory lung diseases. Pharmacol Ther. 2010; Feb. 125(2):286–327.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allergic Rhinitis Mouse Model
- Principles and Application of Mouse Model of Allergic Rhinitis
- Understanding the Mouse Model of Respiratory Allergic Diseases
- The Role of Aviation Medical Examiners in the Diagnosis, Treatment and Aeromedical Assessment of Patients with Allergic Rhinitis
- The Effect of Interleukin-12 on the Immune Response in Allergic Rhinitis Mouse Model