Nutr Res Pract.
2017 Dec;11(6):452-460. 10.4162/nrp.2017.11.6.452.
Acute and 13-week subchronic toxicological evaluations of turanose in mice
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, 52 Ewhayeodae-gil, Seodaemun-gu, Seoul 03760, Korea. yuri.kim@ewha.ac.kr
- 2Department of Food Science and Biotechnology, and Carbohydrate Bioproduct Research Center, Sejong University, 209 Neungdong-ro, Gwangjin-gu, Seoul 05006, Korea. shyoo@sejong.ac.kr
- 3Department of Biomedical Laboratory Science, Eulji University, Seongnam, Gyunggi 13135, Korea.
- KMID: 2395299
- DOI: http://doi.org/10.4162/nrp.2017.11.6.452
Abstract
- BACKGROUND/OBJECTIVES
Turanose, α-D-glucosyl-(1→3)-α-D-fructose, is a sucrose isomer which naturally exists in honey. To evaluate toxicity of turanose, acute and subchronic oral toxicity studies were conducted with ICR mice.
MATERIALS AND METHODS
For the acute oral toxicity study, turanose was administered as a single oral dose [10 g/kg body weight (b.w.)]. In the subchronic toxicity study, ICR mice were administered 0, 1.75, 3.5, and 7 g/kg b.w. doses of turanose daily for 13 weeks.
RESULTS
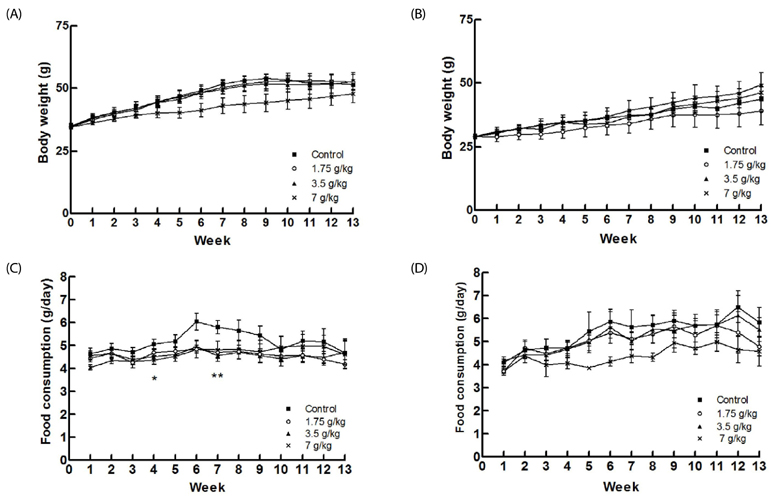
No signs of acute toxicity, including abnormal behavior, adverse effect, or mortality, were observed over the 14-day study period. In addition, no changes in body weight or food consumption were observed and the median lethal dose (LDâ‚…â‚€) for oral intake of turanose was determined to be greater than 10 g/kg b.w. General clinical behavior, changes in body weight and food consumption, absolute and relative organ weights, and mortality were not affected in any of the treatment group for 13 weeks. These doses also did not affect the macroscopic pathology, histology, hematology, and blood biochemical analysis of the mice examined.
CONCLUSION
No toxicity was observed in the acute and 13-week subchronic oral toxicology studies that were conducted with ICR mice. Furthermore, the no-observed-adverse-effect level is greater than 7 g/kg/day for both male and female ICR mice.
MeSH Terms
Figure
Reference
-
1. Augustin LS, Franceschi S, Jenkins DJ, Kendall CW, La Vecchia C. Glycemic index in chronic disease: a review. Eur J Clin Nutr. 2002; 56:1049–1071.
Article2. Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health--a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr. 2008; 87:258S–268S.
Article3. Holub I, Gostner A, Theis S, Nosek L, Kudlich T, Melcher R, Scheppach W. Novel findings on the metabolic effects of the low glycaemic carbohydrate isomaltulose (Palatinose). Br J Nutr. 2010; 103:1730–1737.
Article4. Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006; 84:274–288.
Article5. White JW, Hoban N. Composition of honey. IV. Identification of the disaccharides. Arch Biochem Biophys. 1959; 80:386–392.
Article6. Shibuya T, Mandai T, Kubota M, Fukuda S, Kurimoto M, Tsujisaka Y. Production of turanose by cyclomaltodextrin glucanotransferase from Bacillus stearothermophilus. J Appl Glycosci. 2004; 51:223–227.
Article7. Park MO, Lee BH, Lim E, Lim JY, Kim Y, Park CS, Lee HG, Kang HK, Yoo SH. Enzymatic process for high-yield turanose production and its potential property as an adipogenesis regulator. J Agric Food Chem. 2016; 64:4758–4764.
Article8. Wang R, Bae JS, Kim JH, Kim BS, Yoon SH, Park CS, Yoo SH. Development of an efficient bioprocess for turanose production by sucrose isomerisation reaction of amylosucrase. Food Chem. 2012; 132:773–779.
Article9. Pikis A, Immel S, Robrish SA, Thompson J. Metabolism of sucrose and its five isomers by Fusobacterium mortiferum. Microbiology. 2002; 148:843–852.
Article10. Thompson J, Robrish SA, Pikis A, Brust A, Lichtenthaler FW. Phosphorylation and metabolism of sucrose and its five linkageisomeric alpha-D-glucosyl-D-fructoses by Klebsiella pneumoniae. Carbohydr Res. 2001; 331:149–161.11. Grenby TH. Advances in Sweeteners. London: Blackie Academic & Professional;1996.12. Dahlqvist A. Characterization of hog intestinal invertase as a glucosido-invertase. III. Specificity of purified invertase. Acta Chem Scand. 1960; 14:63–71.
Article13. Organisation for Economic Co-operation and Development. OECD guideline for testing for chemicals: acute oral toxicity-fixed dose procedure [Internet]. Paris: Organisation for Economic Co-operation and Development;2016. cited 2017 May 6. Available from: http://www.oecd-ilibrary.org/environment/test-no-420-acute-oral-toxicity-fixed-dose-procedure_9789264070943-en.14. Lina BA, Jonker D, Kozianowski G. Isomaltulose (Palatinose): a review of biological and toxicological studies. Food Chem Toxicol. 2002; 40:1375–1381.
Article15. Jonker D, Lina BA, Kozianowski G. 13-Week oral toxicity study with isomaltulose (Palatinose) in rats. Food Chem Toxicol. 2002; 40:1383–1389.
Article16. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008; 22:659–661.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of Inflammation by Sucrose Isomer, Turanose, in Raw 264.7 Cells
- 13-week subchronic toxicity study of a novel ginsenoside composition from ginseng leaves in rats
- Toxicological Evaluations of Rare Earths and Their Health Impacts to Workers: A Literature Review
- Preclinical safety assessment of Angelica acutiloba using a 13-week repeated dose oral toxicity study in rats
- Subchronic Inhalation Toxicity Study of n-pentane in Rats