Lab Anim Res.
2017 Sep;33(3):223-230. 10.5625/lar.2017.33.3.223.
Preclinical safety assessment of Angelica acutiloba using a 13-week repeated dose oral toxicity study in rats
- Affiliations
-
- 1Department of Biotechnology, The Catholic University of Korea, Bucheon, Gyeonggi-do, Korea.
- 2Department of Experimental Animal Research, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea. casache@snu.ac.kr
- 3Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Urology, Seoul National University College of Medicine, Seoul, Korea.
- 5Biomedical Center for Animal Resource and Development, Seoul National University College of Medicine, Seoul, Korea. bckang@snu.ac.kr
- 6Graduate School of Translational Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 7Designed Animal and Transplantation Research Institute, Institute of GreenBio Science Technology, Seoul National University, Pyeongchang-gun, Gangwon-do, Korea.
- KMID: 2391095
- DOI: http://doi.org/10.5625/lar.2017.33.3.223
Abstract
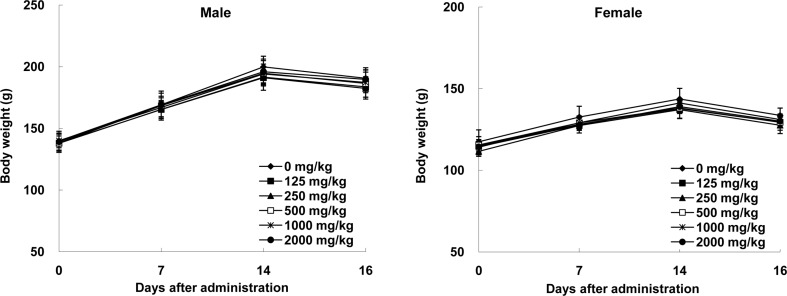
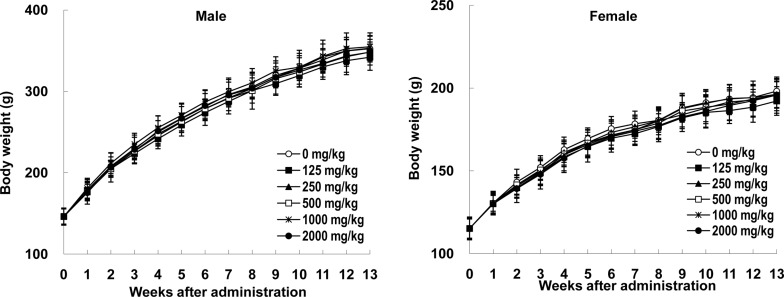
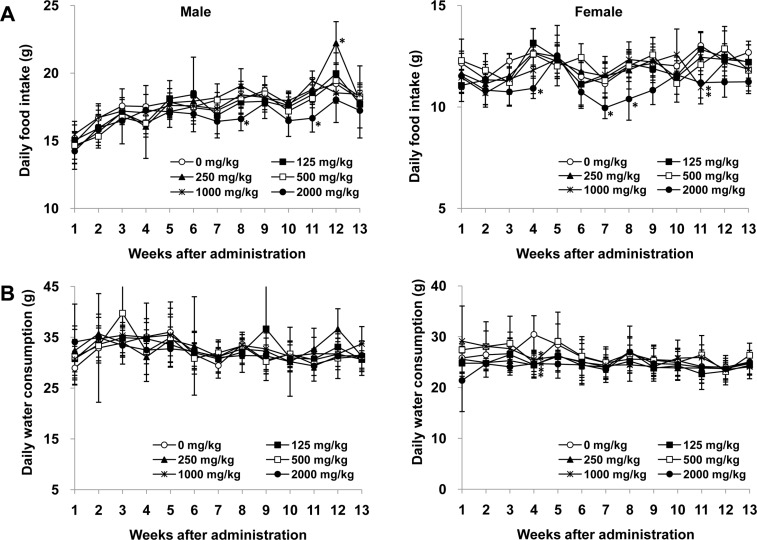
- Angelica acutiloba (AA), a Japanese species of Danggui, has been used worldwide as a traditional herbal medicine with several bioactivities including anti-diabetic, anti-allergic, anti-inflammatory, anti-tumor, and anti-obesity. However, there is lack of toxicological data available to evaluate potential long-term toxicity and the no-observed-adverse-effect level (NOAEL) of AA extract in accordance with the test guidelines published by the Organization for Economic Cooperation and Development. In the 14-day repeat-dose toxicity study, no adverse effects on mortality, body weight change, clinical signs, and organ weights was found following repeat oral administration to rats for 14 days (125, 250, 500, 1000, and 2000 mg/kg body weight), leading that 2000 mg/kg is the highest recommended dose of AA extract for the 13-week repeat-dose oral toxicity study. In the 13-week repeat-dose oral toxicity study, the AA extract was orally administered to groups of rats for 13 weeks (125, 250, 500, 1000, and 2000 mg/kg body weight) to compare between control and AA extract groups. The administration of AA extract did not produce mortality or remarkable clinical signs during this 13-week study. And, the data revealed that there were no significant differences in food/water consumption, body weight, hematological parameters, clinical chemistry parameters, gross macroscopic findings, organ weight and histopathology in comparison to the control group. On the basis of these results, the subchronic NOAEL of the AA extract was more than 2000 mg/kg/day when tested in rats. And, the AA extract is considered safe to use orally as a traditional herbal medicine.
Keyword
MeSH Terms
Figure
Reference
-
1. Park MA, Sim MJ, Kim YC. Anti-Photoaging Effects of Angelica acutiloba Root Ethanol Extract in Human Dermal Fibroblasts. Toxicol Res. 2017; 33(2):125–134. PMID: 28503261.2. Sheng YX, Li L, Wang Q, Guo HZ, Guo DA. Simultaneous determination of gallic acid, albiflorin, paeoniflorin, ferulic acid and benzoic acid in Si-Wu decoction by high-performance liquid chromatography DAD method. J Pharm Biomed Anal. 2005; 37(4):805–810. PMID: 15797805.
Article3. Wen KC, Huang CY, Lu FL. Determination of baicalin and puerarin in traditional Chinese medicinal preparations by high-performance liquid chromatography. J Chromatogr A. 1993; 631:241–250.
Article4. Zhao KJ, Dong TT, Tu PF, Song ZH, Lo CK, Tsim KW. Molecular genetic and chemical assessment of radix Angelica (Danggui) in China. J Agric Food Chem. 2003; 51(9):2576–2583. PMID: 12696940.
Article5. Lu GH, Chan K, Liang YZ, Leung K, Chan CL, Jiang ZH, Zhao ZZ. Development of high-performance liquid chromatographic fingerprints for distinguishing Chinese Angelica from related umbelliferae herbs. J Chromatogr A. 2005; 1073(1-2):383–392. PMID: 15909545.
Article6. Piao XL, Park JH, Cui J, Kim DH, Yoo HH. Development of gas chromatographic/mass spectrometry-pattern recognition method for the quality control of Korean Angelica. J Pharm Biomed Anal. 2007; 44(5):1163–1167. PMID: 17537609.
Article7. Yamada H, Komiyama K, Kiyohara H, Cyong JC, Hirakawa Y, Otsuka Y. Structural characterization and antitumor activity of a pectic polysaccharide from the roots of Angelica acutiloba. Planta Med. 1990; 56(2):182–186. PMID: 2353065.8. Kiyohara H, Yamada H, Cyong JC, Otsuka Y. Studies on polysaccharides from Angelica acutiloba. V. Molecular aggregation and anti-complementary activity of arabinogalactan from Angelica acutiloba. J Pharmacobiodyn. 1986; 9(4):339–346. PMID: 3735056.
Article9. Kim AR, Lee JJ, Lee MY. Antioxidative effect of Angelica acutiloba Kitagawa ethanol extract. J Life Sci. 2009; 19:117–122.10. Yoon TS, Cheon MS, Lee DY, Moon BC, Lee HW, Choo BK, Kim HK. Effects of root extracts from Angelica gigas and Angelica acutiloba on inflammatory mediators in mouse macrophages. J Appl Biol Chem. 2007; 50(4):264–269.11. Tanaka S, Kano Y, Tabata M, Konoshima M. [Effects of “Toki” (Angelica acutiloba Kitagawa) extracts on writhing and capillary permeability in mice (analgesic and antiinflammatory effects)]. Yakugaku Zasshi. 1971; 91(10):1098–1104. PMID: 5170013.12. Tanaka S, Ikeshiro Y, Tabata M, Konoshima M. Anti-nociceptive substances from the roots of Angelica acutiloba. Arzneimittelforschung. 1977; 27(11):2039–2045. PMID: 579998.13. Lee K, Sohn Y, Lee MJ, Cho HS, Jang MH, Han NY, Shin KW, Kim SH, Cho IH, Bu Y, Jung HS. Effects of Angelica acutiloba on mast cell-mediated allergic reactions in vitro and in vivo. Immunopharmacol Immunotoxicol. 2012; 34(4):571–577. PMID: 22129085.14. Liu IM, Tzeng TF, Liou SS, Chang CJ. Regulation of obesity and lipid disorders by extracts from Angelica acutiloba root in high-fat diet-induced obese rats. Phytother Res. 2012; 26(2):223–230. PMID: 21647998.15. Liu IM, Tzeng TF, Liou SS, Chang CJ. Angelica acutiloba root attenuates insulin resistance induced by high-fructose diet in rats. Phytother Res. 2011; 25(9):1283–1293. PMID: 21308821.16. Joo SS, Park D, Shin S, Jeon JH, Kim TK, Choi YJ, Lee SH, Kim JS, Park SK, Hwang BY, Lee DI, Kim YB. Anti-allergic effects and mechanisms of action of the ethanolic extract of Angelica gigas in dinitrofluorobenzene-induced inflammation models. Environ Toxicol Pharmacol. 2010; 30(2):127–133. PMID: 21787642.
Article17. Sarker SD, Nahar L. Natural medicine: the genus Angelica. Curr Med Chem. 2004; 11(11):1479–1500. PMID: 15180579.18. Uto T, Tung NH, Taniyama R, Miyanowaki T, Morinaga O, Shoyama Y. Anti-inflammatory Activity of Constituents Isolated from Aerial Part of Angelica acutiloba Kitagawa. Phytother Res. 2015; 29(12):1956–1963. PMID: 26463105.
Article19. Markman M. Safety issues in using complementary and alternative medicine. J Clin Oncol. 2002; 20:39S–41S. PMID: 12235223.20. Shin SH, Koo KH, Bae JS, Cha SB, Kang IS, Kang MS, Kim HS, Heo HS, Park MS, Gil GH, Lee JY, Kim KH, Li Y, Lee HK, Song SW, Choi HS, Kang BH, Kim JC. Single and 90-day repeated oral dose toxicity studies of fermented Rhus verniciflua stem bark extract in Sprague-Dawley rats. Food Chem Toxicol. 2013; 55:617–626. PMID: 23416650.
Article21. Che JH, Yun JW, Cho EY, Kim SH, Kim YS, Kim WH, Park JH, Son WC, Kim MK, Kang BC. Toxicologic assessment of Paecilomyces tenuipes in rats: renal toxicity and mutagenic potential. Regul Toxicol Pharmacol. 2014; 70(2):527–534. PMID: 25223566.
Article22. Che JH, Yun JW, Kim YS, Kim SH, You JR, Jang JJ, Kim HC, Kim HH, Kang BC. Genotoxicity and subchronic toxicity of Sophorae radix in rats: hepatotoxic and genotoxic potential. Regul Toxicol Pharmacol. 2015; 71(3):379–387. PMID: 25640205.
Article23. Yun JW, Kim SH, Kim YS, You JR, Kwon E, Jang JJ, Park IA, Kim HC, Kim HH, Che JH, Kang BC. Evaluation of subchronic (13week) toxicity and genotoxicity potential of vinegar-processed Genkwa Flos. Regul Toxicol Pharmacol. 2015; 72(2):386–393. PMID: 25882305.
Article24. Yun JW, Che JH, Kwon E, Kim YS, Kim SH, You JR, Kim WH, Kim HH, Kang BC. Safety evaluation of Angelica gigas: Genotoxicity and 13-weeks oral subchronic toxicity in rats. Regul Toxicol Pharmacol. 2015; 72(3):473–480. PMID: 26032491.
Article25. Ministry of Food and Drug Safety (MFDS). Good laboratory practice regulation for non-clinical laboratory studies. 2005. (Notification no. 2005-79).26. OECD. OECD guideline for testing of chemicals, Test No. 408: repeated dose 90-day oral toxicity study in rodents. 1998.27. Lim KT, Lim V, Chin JH. Subacute oral toxicity study of ethanolic leaves extracts of Strobilanthes crispus in rats. Asian Pac J Trop Biomed. 2012; 2(12):948–952. PMID: 23593574.
Article28. Worasuttayangkurn L, Watcharasit P, Rangkadilok N, Suntararuks S, Khamkong P, Satayavivad J. Safety evaluation of longan seed extract: acute and repeated oral administration. Food Chem Toxicol. 2012; 50(11):3949–3955. PMID: 22902805.
Article29. Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010; 28(5):478–485. PMID: 20458318.
Article30. Chen Y, Brott D, Luo W, Gangl E, Kamendi H, Barthlow H, Lengel D, Fikes J, Kinter L, Valentin JP, Bialecki R. Assessment of cisplatin-induced kidney injury using an integrated rodent platform. Toxicol Appl Pharmacol. 2013; 268(3):352–361. PMID: 23415679.
Article31. Han YD, Song SY, Lee JH, Lee DS, Yoon HC. Multienzyme-modified biosensing surface for the electrochemical analysis of aspartate transaminase and alanine transaminase in human plasma. Anal Bioanal Chem. 2011; 400(3):797–805. PMID: 21359826.
Article32. Ozdil B, Kece C, Cosar A, Akkiz H, Sandikci M. Potential benefits of combined N-acetylcysteine and ciprofloxacin therapy in partial biliary obstruction. J Clin Pharmacol. 2010; 50(12):1414–1419. PMID: 20388917.
Article33. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008; 22(3):659–661. PMID: 17942826.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Didymella acutilobae sp. nov. Causing Leaf Spot and Stem Rot in Angelica acutiloba
- Pharmaceutical Studies on “Dang-Gui†in Korean Journals
- A 13-week repeated dose oral toxicity study on a water extract of Artemisia annua in Sprague-Dawley rat
- Acute and 28-days subacute toxicity studies of Gαq-RGS2 signaling inhibitor
- Acute Oral or Dermal and Repeated Dose 90-Day Oral Toxicity of Tetrasodium Pyrophosphate in Spraque Dawley (SD) Rats