Effects of Lobeglitazone, a New Thiazolidinedione, on Osteoblastogenesis and Bone Mineral Density in Mice
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. limsoo@snu.ac.kr
- KMID: 2389821
- DOI: http://doi.org/10.3803/EnM.2017.32.3.389
Abstract
- BACKGROUND
Bone strength is impaired in patients with type 2 diabetes mellitus despite an increase in bone mineral density (BMD). Thiazolidinedione (TZD), a peroxisome proliferator activated receptor γ agonist, promotes adipogenesis, and suppresses osteoblastogenesis. Therefore, its use is associated with an increased risk of fracture. The aim of this study was to examine the in vitro and in vivo effects of lobeglitazone, a new TZD, on bone.
METHODS
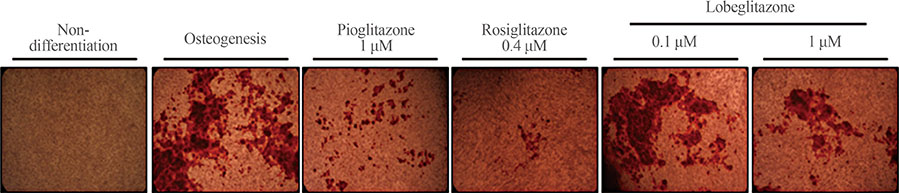
MC3T3E1 and C3H10T1/2 cells were cultured in osteogenic medium and exposed to lobeglitazone (0.1 or 1 µM), rosiglitazone (0.4 µM), or pioglitazone (1 µM) for 10 to 14 days. Alkaline phosphatase (ALP) activity, Alizarin red staining, and osteoblast marker gene expression were analyzed. For in vivo experiments, 6-month-old C57BL/6 mice were treated with vehicle, one of two doses of lobeglitazone, rosiglitazone, or pioglitazone. BMD was assessed using a PIXImus2 instrument at the baseline and after 12 weeks of treatment.
RESULTS
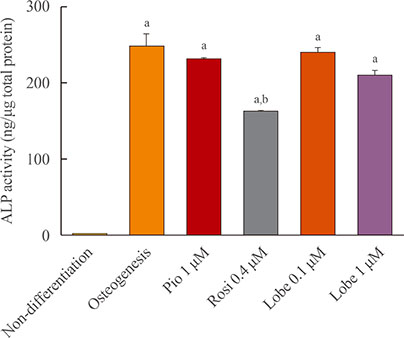
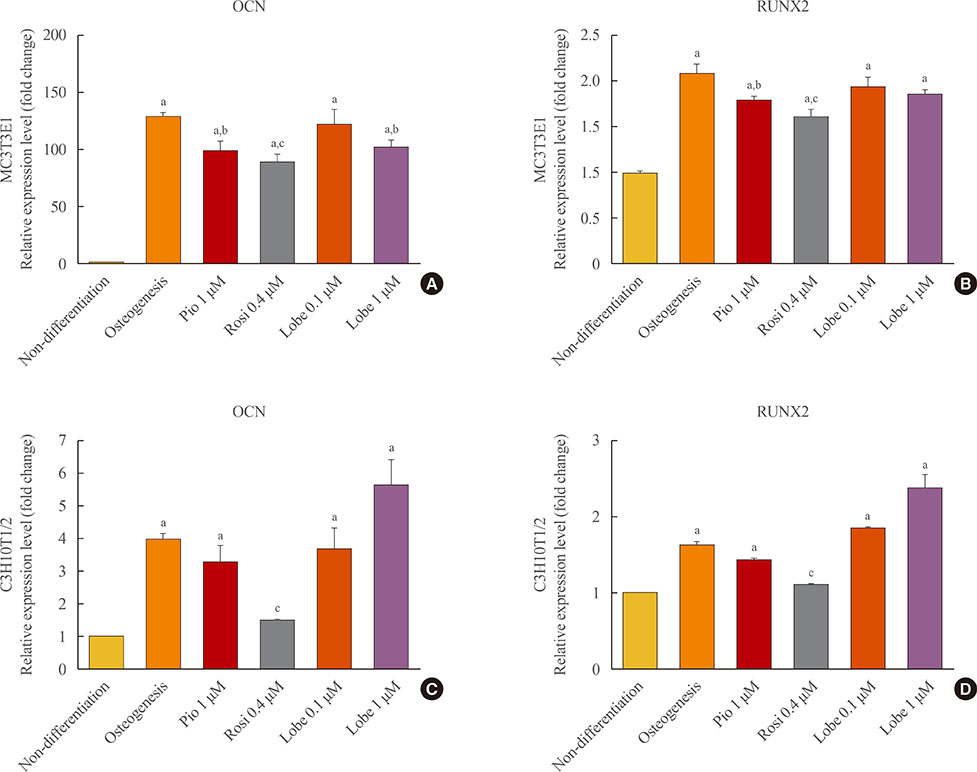
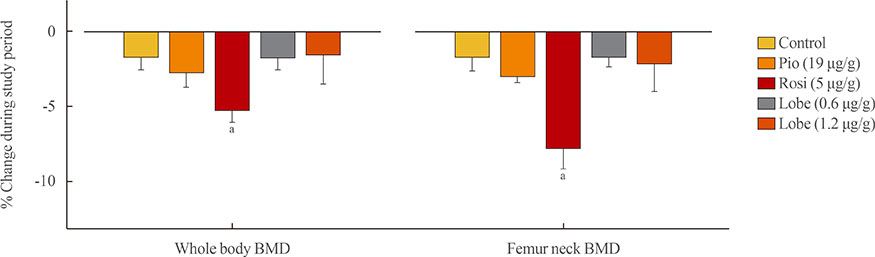
As expected, in vitro experiments showed that ALP activity was suppressed and the mRNA expression of osteoblast marker genes RUNX2 (runt-related transcription factor 2) and osteocalcin was significantly attenuated after rosiglitazone treatment. By contrast, lobeglitazone at either dose did not inhibit these variables. Rosiglitazone-treated mice showed significantly accelerated bone loss for the whole bone and femur, but BMD did not differ significantly between the lobeglitazone-treated and vehicle-treated mice.
CONCLUSION
These findings suggest that lobeglitazone has no detrimental effects on osteoblast biology and might not induce side effects in the skeletal system.
MeSH Terms
-
Adipogenesis
Alkaline Phosphatase
Animals
Biology
Bone and Bones
Bone Density*
Diabetes Mellitus, Type 2
Femur
Gene Expression
Humans
In Vitro Techniques
Infant
Mice*
Osteoblasts
Osteocalcin
Peroxisomes
RNA, Messenger
Thiazolidinediones
Transcription Factors
Alkaline Phosphatase
Osteocalcin
RNA, Messenger
Thiazolidinediones
Transcription Factors
Figure
Cited by 3 articles
-
Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
Jaehyun Bae, Taegyun Park, Hyeyoung Kim, Minyoung Lee, Bong-Soo Cha
Diabetes Metab J. 2021;45(3):326-336. doi: 10.4093/dmj.2020.0272.Comparison of the Effects of Various Antidiabetic Medication on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus
Jeonghoon Ha, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Ki-Ho Song, Seung Hyun Ko, Moo Il Kang, Sung Dae Moon, Ki-Hyun Baek
Endocrinol Metab. 2021;36(4):895-903. doi: 10.3803/EnM.2021.1026.A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
Bo-Yeon Kim, Hyuk-Sang Kwon, Suk Kyeong Kim, Jung-Hyun Noh, Cheol-Young Park, Hyeong-Kyu Park, Kee-Ho Song, Jong Chul Won, Jae Myung Yu, Mi Young Lee, Jae Hyuk Lee, Soo Lim, Sung Wan Chun, In-Kyung Jeong, Choon Hee Chung, Seung Jin Han, Hee-Seok Kim, Ju-Young Min, Sungrae Kim
Diabetes Metab J. 2022;46(6):855-865. doi: 10.4093/dmj.2021.0264.
Reference
-
1. Overbeek JA, Heintjes EM, Prieto-Alhambra D, Blin P, Lassalle R, Hall GC, et al. Type 2 diabetes mellitus treatment patterns across Europe: a population-based multi-database study. Clin Ther. 2017; 39:759–770.2. Stolar MW, Hoogwerf BJ, Gorshow SM, Boyle PJ, Wales DO. Managing type 2 diabetes: going beyond glycemic control. J Manag Care Pharm. 2008; 14:5 Suppl B. S2–S19.3. Tran L, Zielinski A, Roach AH, Jende JA, Householder AM, Cole EE, et al. Pharmacologic treatment of type 2 diabetes: oral medications. Ann Pharmacother. 2015; 49:540–556.4. Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004; 351:1106–1118.5. Grey A, Bolland M, Fenwick S, Horne A, Gamble G, Drury PL, et al. The skeletal effects of pioglitazone in type 2 diabetes or impaired glucose tolerance: a randomized controlled trial. Eur J Endocrinol. 2013; 170:255–262.6. Kahn BB, McGraw TE. Rosiglitazone, PPARγ, and type 2 diabetes. N Engl J Med. 2010; 363:2667–2669.7. Ksiazek K. A comprehensive review on mesenchymal stem cell growth and senescence. Rejuvenation Res. 2009; 12:105–116.8. Wan Y. PPARγ in bone homeostasis. Trends Endocrinol Metab. 2010; 21:722–728.9. Patel JJ, Butters OR, Arnett TR. PPAR agonists stimulate adipogenesis at the expense of osteoblast differentiation while inhibiting osteoclast formation and activity. Cell Biochem Funct. 2014; 32:368–377.10. Billington EO, Grey A, Bolland MJ. The effect of thiazolidinediones on bone mineral density and bone turnover: systematic review and meta-analysis. Diabetologia. 2015; 58:2238–2246.11. Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005; 146:1226–1235.12. Bilezikian JP, Josse RG, Eastell R, Lewiecki EM, Miller CG, Wooddell M, et al. Rosiglitazone decreases bone mineral density and increases bone turnover in postmenopausal women with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013; 98:1519–1528.13. Fukunaga T, Zou W, Rohatgi N, Colca JR, Teitelbaum SL. An insulin-sensitizing thiazolidinedione, which minimally activates PPARγ, does not cause bone loss. J Bone Miner Res. 2015; 30:481–488.14. Lazarenko OP, Rzonca SO, Suva LJ, Lecka-Czernik B. Netoglitazone is a PPAR-gamma ligand with selective effects on bone and fat. Bone. 2006; 38:74–84.15. Liu C, Feng T, Zhu N, Liu P, Han X, Chen M, et al. Identification of a novel selective agonist of PPARγ with no promotion of adipogenesis and less inhibition of osteoblastogenesis. Sci Rep. 2015; 5:9530.16. Sellmeyer DE, Civitelli R, Hofbauer LC, Khosla S, Lecka-Czernik B, Schwartz AV. Skeletal metabolism, fracture risk, and fracture outcomes in type 1 and type 2 diabetes. Diabetes. 2016; 65:1757–1766.17. Schwartz AV. Diabetes, bone and glucose-lowering agents: clinical outcomes. Diabetologia. 2017; 60:1170–1179.18. Bae KH, Seo JB, Jung YA, Seo HY, Kang SH, Jeon HJ, et al. Lobeglitazone, a novel peroxisome proliferator-activated receptor γ agonist, attenuates renal fibrosis caused by unilateral ureteral obstruction in mice. Endocrinol Metab (Seoul). 2017; 32:115–123.19. Osman I, Segar L. Pioglitazone, a PPARγ agonist, attenuates PDGF-induced vascular smooth muscle cell proliferation through AMPK-dependent and AMPK-independent inhibition of mTOR/p70S6K and ERK signaling. Biochem Pharmacol. 2016; 101:54–70.20. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016; 374:1321–1331.21. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomized controlled trial. Lancet. 2005; 366:1279–1289.22. Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab. 2012; 23:205–215.23. Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003; 423:349–355.24. Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008; 473:201–209.25. Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone. 2009; 44:1026–1033.26. Crossno JT Jr, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006; 116:3220–3228.27. Berberoglu Z, Yazici AC, Demirag NG. Effects of rosiglitazone on bone mineral density and remodelling parameters in postmenopausal diabetic women: a 2-year follow-up study. Clin Endocrinol (Oxf). 2010; 73:305–312.28. Bone HG, Lindsay R, McClung MR, Perez AT, Raanan MG, Spanheimer RG. Effects of pioglitazone on bone in postmenopausal women with impaired fasting glucose or impaired glucose tolerance: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2013; 98:4691–4701.29. Li R, Xu W, Luo S, Xu H, Tong G, Zeng L, et al. Effect of exenatide, insulin and pioglitazone on bone metabolism in patients with newly diagnosed type 2 diabetes. Acta Diabetol. 2015; 52:1083–1091.30. Lee HW, Kim BY, Ahn JB, Kang SK, Lee JH, Shin JS, et al. Molecular design, synthesis, and hypoglycemic and hypolipidemic activities of novel pyrimidine derivatives having thiazolidinedione. Eur J Med Chem. 2005; 40:862–874.31. Kim BY, Ahn JB, Lee HW, Kang SK, Lee JH, Shin JS, et al. Synthesis and biological activity of novel substituted pyridines and purines containing 2,4-thiazolidinedione. Eur J Med Chem. 2004; 39:433–447.32. Kim SG, Kim DM, Woo JT, Jang HC, Chung CH, Ko KS, et al. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial. PLoS One. 2014; 9:e92843.33. Kim SH, Kim SG, Kim DM, Woo JT, Jang HC, Chung CH, et al. Safety and efficacy of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 52 weeks: an open-label extension study. Diabetes Res Clin Pract. 2015; 110:e27–e30.34. Lim S, Lee KS, Lee JE, Park HS, Kim KM, Moon JH, et al. Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015; 243:107–119.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Lobeglitazone, a Novel Thiazolidinedione, on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus over 52 Weeks
- Changes in the Bone Mineral Density of Femur Neck and Total Hip Over a 52-Week Treatment with Lobeglitazone
- Effects of teriparatide on bone in autochthonous transgenic model mice for diabetes mellitus (Akita mice)
- The relationship of maturation value of vaginal epithelium and bone mineral density in postmenopausal women
- Anti-Diabetic Medications and Osteoporosis