Korean J Physiol Pharmacol.
2017 Sep;21(5):457-464. 10.4196/kjpp.2017.21.5.457.
Effect of etoricoxib on experimental oxidative testicular ischemia-reperfusion damage in rats induced with torsion-detorsion
- Affiliations
-
- 1Department of Urology, Faculty of Medicine, Ataturk University, Erzurum 25240, Turkey.
- 2Department of Urology, Bayburt State Hospital, Bayburt 69010, Turkey.
- 3Department of Urology, Bilecik State Hospital, Bilecik 11100, Turkey.
- 4Department of Pharmacology, Faculty of Medicine, Erzincan University, Erzincan 24100, Turkey. durdualtuner@hotmail.com
- 5Department of Pathology, Mengucek Gazi Education and Research Hospital, Erzincan 24100, Turkey.
- 6Department of Urology, Faculty of Medicine, Erzincan University, Erzincan 24100, Turkey.
- KMID: 2388691
- DOI: http://doi.org/10.4196/kjpp.2017.21.5.457
Abstract
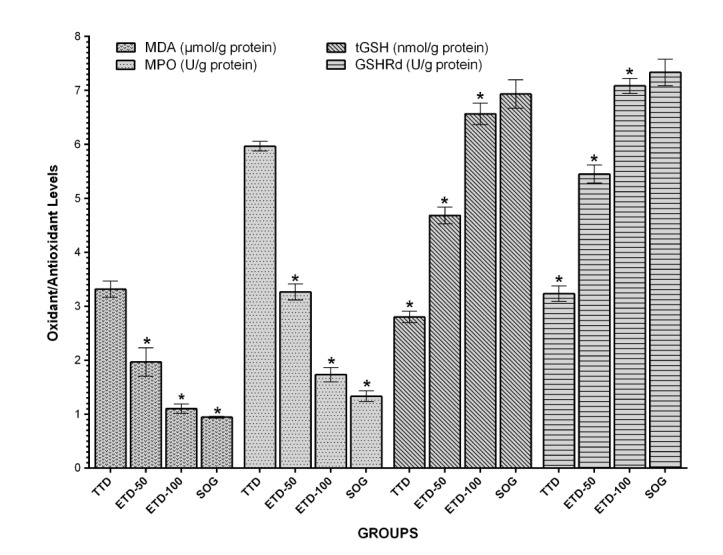
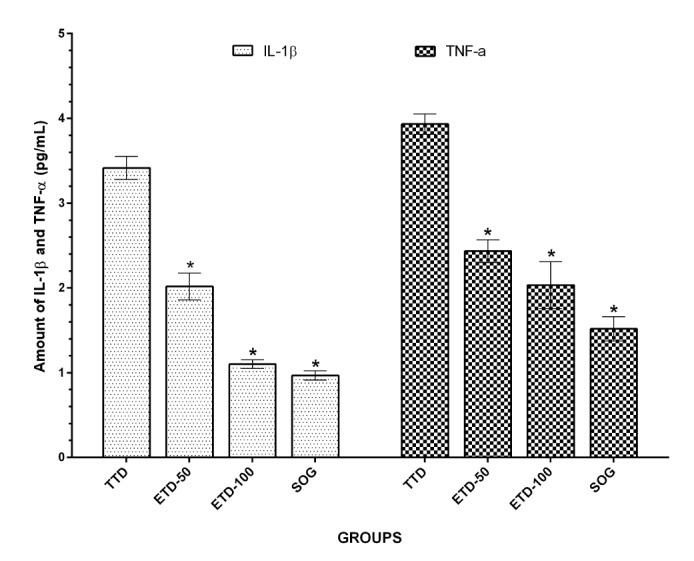
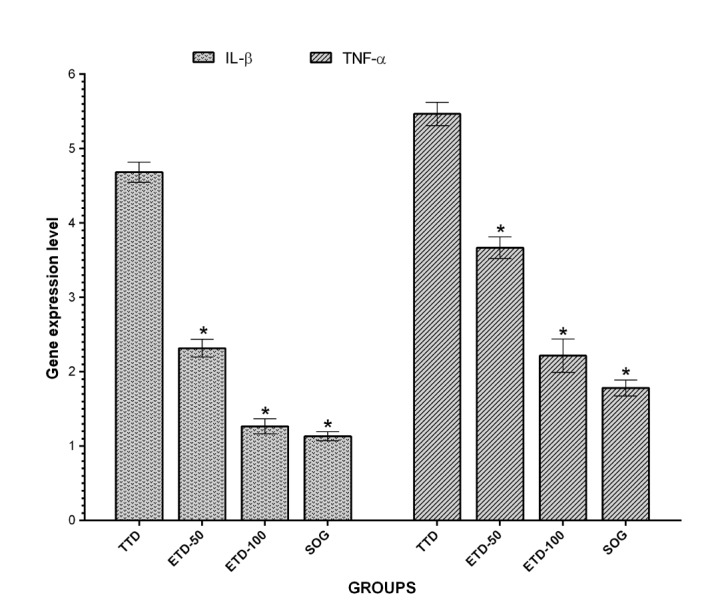
- Etoricoxib features antioxidant and anti-inflammatory properties concomitantly, suggesting that it may be beneficial in testicular ischemia reperfusion (I/R) damage. Our aim is to investigate the effects of etoricoxib on testicular I/R damage induced with torsion-detorsion (TD). The etoricoxib + torsion-detorsion (ETD) groups of animals were given etoricoxib in 50 and 100 mg/kg of body weight (ETD-50 and ETD-100), while the testes torsion-detorsion (TTD) and sham operation rat group (SOG) animals were given single oral doses of distilled water as a solvent. TTD, ETD-50 and ETD-100 groups were subjected to 720° degrees torsion for four hours, and detorsion for four hours. The SOG group was not subjected to this procedure. Biochemical, gene expression and histopathological analyses were carried out on the testicular tissues. The levels of malondialdehyde (MDA), myeloperoxidase (MPO), interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) were significantly higher, and the levels of total glutathione (tGSH) and glutathione reductase (GSHRd) were significantly lower in the TTD group, compared to the ETD-50, ETD-100 and SOG groups. Etoricoxib at a dose of 100 mg/kg better prevented I/R damage than the 50 mg/kg dose. Etoricoxib may be useful in clinical practice in the reduction of I/R damage on testes caused by torsion-detorsion.
MeSH Terms
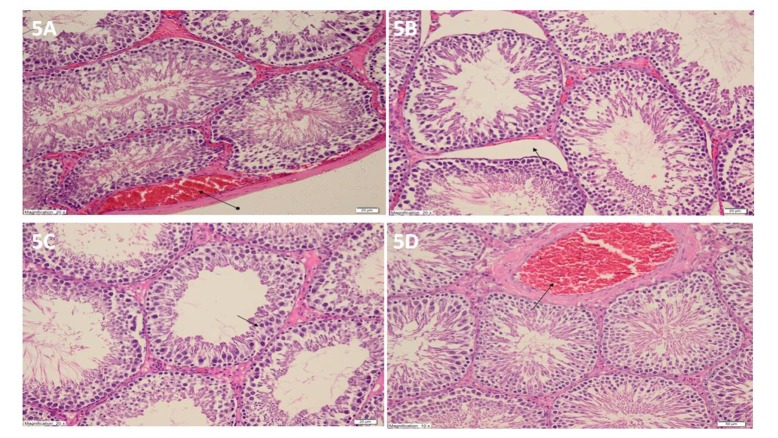
Figure
Reference
-
1. Yapca OE, Borekci B, Suleyman H. Ischemia-reperfusion damage. Eurasian J Med. 2013; 45:126–127. PMID: 25610264.
Article2. Parks DA, Granger DN. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol. 1983; 245:G285–G289. PMID: 6309018.
Article3. Del Maestro RF. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl. 1980; 492:153–168. PMID: 6261528.4. Tuglu D, Yuvanc E, Yilmaz E, Gencay IY, Atasoy P, Kisa U, Batislam E. The antioxidant effect of dexmedetomidine on testicular ischemia-reperfusion injury. Acta Cir Bras. 2015; 30:414–421. PMID: 26108030.
Article5. Cvetkovic T, Stankovic J, Najman S, Pavlovic D, Stokanovic D, Vlajkovic S, Dakovic-Bjelakovic M, Cukuranovic J, Zivkovic V, Stefanovic V. Oxidant and antioxidant status in experimental rat testis after testicular torsion/detorsion. Int J Fertil Steril. 2015; 9:121–128. PMID: 25918600.6. Shih HJ, Yen JC, Chiu AW, Chow YC, Pan WH, Wang TY, Huang CJ. FTY720 mitigates torsion/detorsion-induced testicular injury in rats. J Surg Res. 2015; 196:325–331. PMID: 25862489.
Article7. Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007; 376:1–43. PMID: 18038125.
Article8. Beutler B. Neo-ligands for innate immune receptors and the etiology of sterile inflammatory disease. Immunol Rev. 2007; 220:113–128. PMID: 17979843.
Article9. Coley BD. The acute pediatric scrotum. Ultrasound Clinics. 2006; 1:485–496.
Article10. Riendeau D, Percival MD, Brideau C, Charleson S, Dubé D, Ethier D, Falgueyret JP, Friesen RW, Gordon R, Greig G, Guay J, Mancini J, Ouellet M, Wong E, Xu L, Boyce S, Visco D, Girard Y, Prasit P, Zamboni R, Rodger IW, Gresser M, Ford-Hutchinson AW, Young RN, Chan CC. Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther. 2001; 296:558–566. PMID: 11160644.11. Gatti D, Adami S. Coxibs: a significant therapeutic opportunity. Acta Biomed. 2010; 81:217–224. PMID: 22530460.12. Suleyman B, Albayrak A, Kurt N, Demirci E, Gundogdu C, Aksoy M. The effect of etoricoxib on kidney ischemia-reperfusion injury in rats: a biochemical and immunohistochemical assessment. Int Immunopharmacol. 2014; 23:179–185. PMID: 25068826.
Article13. Yapca OE, Turan MI, Yilmaz I, Salman S, Gulapoglu M, Suleyman H. Benefits of the antioxidant and anti-inflammatory activity of etoricoxib in the prevention of ovarian ischemia/reperfusion injury induced experimentally in rats. J Obstet Gynaecol Res. 2014; 40:1674–1679. PMID: 24888933.
Article14. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358. PMID: 36810.
Article15. Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982; 78:206–209. PMID: 6276474.
Article16. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968; 25:192–205. PMID: 4973948.
Article17. Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985; 113:484–490. PMID: 3003504.18. Ciftci AO, Senocak ME, Tanyel FC, Büyükpamukçu N. Clinical predictors for differential diagnosis of acute scrotum. Eur J Pediatr Surg. 2004; 14:333–338. PMID: 15543483.
Article19. Mushtaq I, Fung M, Glasson MJ. Retrospective review of paediatric patients with acute scrotum. ANZ J Surg. 2003; 73:55–58. PMID: 12534742.
Article20. Heindel RM, Pakyz RE, Reinking LN, Cosentino MJ. The effect of various degrees of unilateral spermatic cord torsion on fertility in the rat. J Urol. 1990; 144:366–369. PMID: 2374208.
Article21. Janetschek G, Schreckenberg F, Grimm W, Marberger M. Hemodynamic effects of experimental testicular torsion. Urol Res. 1987; 15:303–306. PMID: 3686761.
Article22. Turner TT. Acute experimental testicular torsion. No effect on the contralateral testis. J Androl. 1985; 6:65–72. PMID: 3972720.
Article23. Mertoğlu C, Senel U, Cayli S, Tas U, Küskü Kiraz Z, Özyurt H. Protective role of methylprednisolone and heparin in ischaemic-reperfusion injury of the rat testicle. Andrologia. 2016; 48:737–744. PMID: 26626546.24. Zhou XL, Yang QS, Ni SZ, Tu XP, Zhao Y, Xu B, Pan ZQ, Shen J. Protective effects of lipoxin A4 in testis injury following testicular torsion and detorsion in rats. Mediators Inflamm. 2014; 2014:898056. PMID: 24904198.
Article25. Sılay MS, Toklu H2, Özağarı A, Aydın M, Tetik ş, şener G, Miroğlu C, Kendirci M. Montelukast prevents testes against ischemia-reperfusion injury through suppression of iNOS expression. Turk J Urol. 2014; 40:221–227. PMID: 26328182.26. Ormrod DJ, Harrison GL, Miller TE. Inhibition of neutrophil myeloperoxidase activity by selected tissues. J Pharmacol Methods. 1987; 18:137–142. PMID: 3041120.
Article27. Kisaoglu A, Borekci B, Yapca OE, Bilen H, Suleyman H. Tissue damage and oxidant/antioxidant balance. Eurasian J Med. 2013; 45:47–49. PMID: 25610248.
Article28. Ozturk H, Ozturk H, Gideroglu K, Terzi H, Bugdayci G. Montelukast protects against testes ischemia/reperfusion injury in rats. Can Urol Assoc J. 2010; 4:174–179. PMID: 20514280.
Article29. Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001; 54:176–186. PMID: 11253127.
Article30. Kinnula VL, Pääkkö P, Soini Y. Antioxidant enzymes and redox regulating thiol proteins in malignancies of human lung. FEBS Lett. 2004; 569:1–6. PMID: 15225599.
Article31. Lysiak JJ. The role of tumor necrosis factor-alpha and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod Biol Endocrinol. Reprod Biol Endocrinol. 2004; 2:9. PMID: 15012831.32. Lysiak JJ, Nguyen QA, Kirby JL, Turner TT. Ischemia-reperfusion of the murine testis stimulates the expression of proinflammatory cytokines and activation of c-jun N-terminal kinase in a pathway to E-selectin expression. Biol Reprod. 2003; 69:202–210. PMID: 12620934.33. Taati M, Moghadasi M, Dezfoulian O, Asadian P, Zendehdel M. Effects of Ghrelin on germ cell apoptosis and proinflammatory cytokines production in Ischemia-reperfusion of the rat testis. Iran J Reprod Med. 2015; 13:85–92. PMID: 25999997.34. Kunak CS, Kukula O, Mutlu E, Genç F, Peker GG, Kuyrukluyıldız U, Binici O, Altuner D, Alp HH. The effect of Etoricoxib on hepatic ischemia-reperfusion injury in rats. Oxid Med Cell Longev. 2015; 2015:598162. PMID: 26236425.
Article35. Sil S, Ghosh T. Role of cox-2 mediated neuroinflammation on the neurodegeneration and cognitive impairments in colchicine induced rat model of Alzheimer's disease. J Neuroimmunol. 2016; 291:115–124. PMID: 26857505.
Article37. Semenzato G. Tumour necrosis factor: a cytokine with multiple biological activities. Br J Cancer. 1990; 61:354–361. PMID: 2183871.
Article38. Hasturk A, Atalay B, Calisaneller T, Ozdemir O, Oruckaptan H, Altinors N. Analysis of serum pro-inflammatory cytokine levels after rat spinal cord ischemia/reperfusion injury and correlation with tissue damage. Turk Neurosurg. 2009; 19:353–359. PMID: 19847755.39. Chi KK, Zhang WH, Chen Z, Cui Y, He W, Wang SG, Zhang C, Chen J, Wang GC. Comparison of quercetin and resveratrol in the prevention of injury due to testicular torsion/detorsion in rats. Asian J Androl. 2015; 18:908–912.
Article40. Minutoli L, Antonuccio P, Squadrito F, Bitto A, Nicotina PA, Fazzari C, Polito F, Marini H, Bonvissuto G, Arena S, Morgia G, Romeo C, Caputi AP, Altavilla D. Effects of polydeoxyribonucleotide on the histological damage and the altered spermatogenesis induced by testicular ischaemia and reperfusion in rats. Int J Androl. 2012; 35:133–144. PMID: 21651579.
Article41. Read MA, Whitley MZ, Gupta S, Pierce JW, Best J, Davis RJ, Collins T. Tumor necrosis factor alpha-induced E-selectin expression is activated by the nuclear factor-kappaB and c-JUN N-terminal kinase/p38 mitogen-activated protein kinase pathways. J Biol Chem. 1997; 272:2753–2761. PMID: 9006914.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of testicular torsion on the contralateral testis in rats
- The Mechanism of Damage to the Contralateral Testis Following Testicular Torsion and Detorsion in Rats and the Effect of Allopurinol Administration
- The Effect of Free Radical Scavengers on Reperfusion Injury after Testicular Torsion
- Twist and Shout: A Clinical and Experimental Review of Testicular Torsion
- The Effect of Testicular Torsion on the Histologic Findings and Apoptosis of the Contralateral Testis at Various Ages in Rats