Transl Clin Pharmacol.
2017 Jun;25(2):85-92. 10.12793/tcp.2017.25.2.85.
Pharmacokinetic characteristics of fluticasone, salmeterol and tiotropium after concurrent inhalation
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul 03080, Republic of Korea. ijjang@snu.ac.kr
- 2Hanmi Pharmaceutical Co., Ltd., Seoul 05545, Republic of Korea.
- KMID: 2386765
- DOI: http://doi.org/10.12793/tcp.2017.25.2.85
Abstract
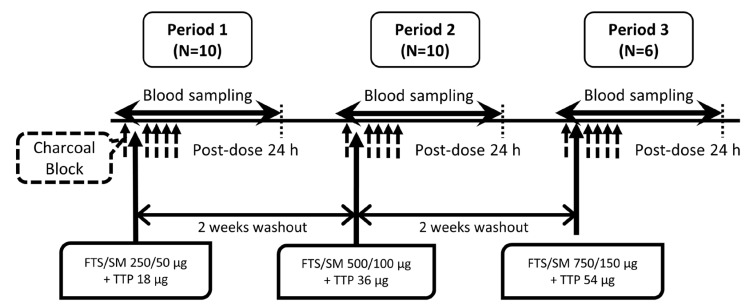
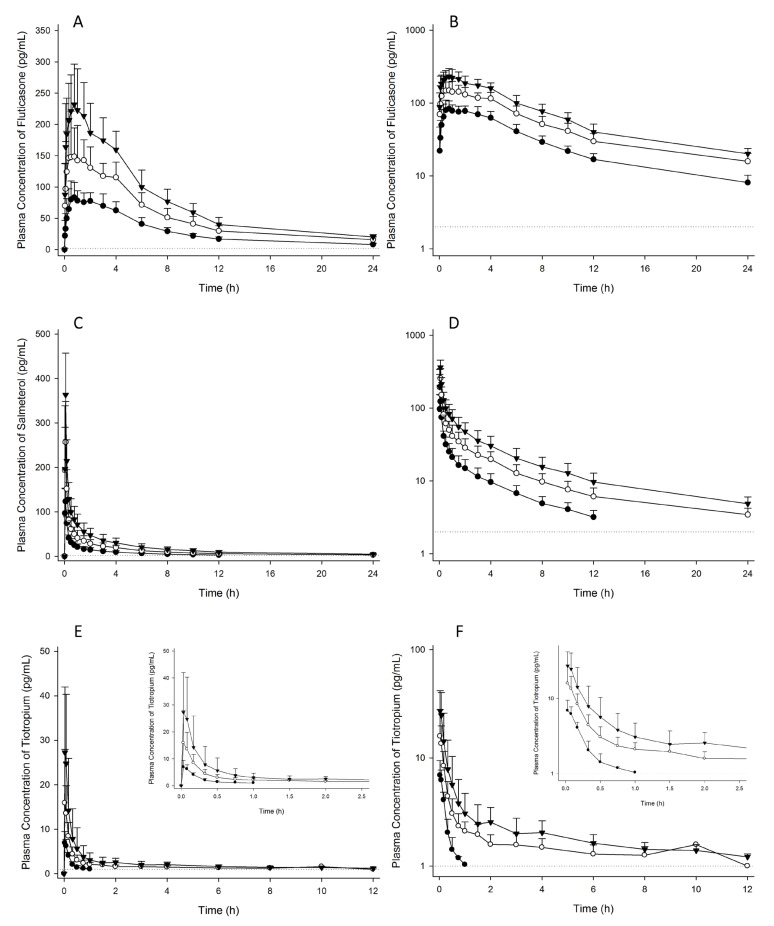
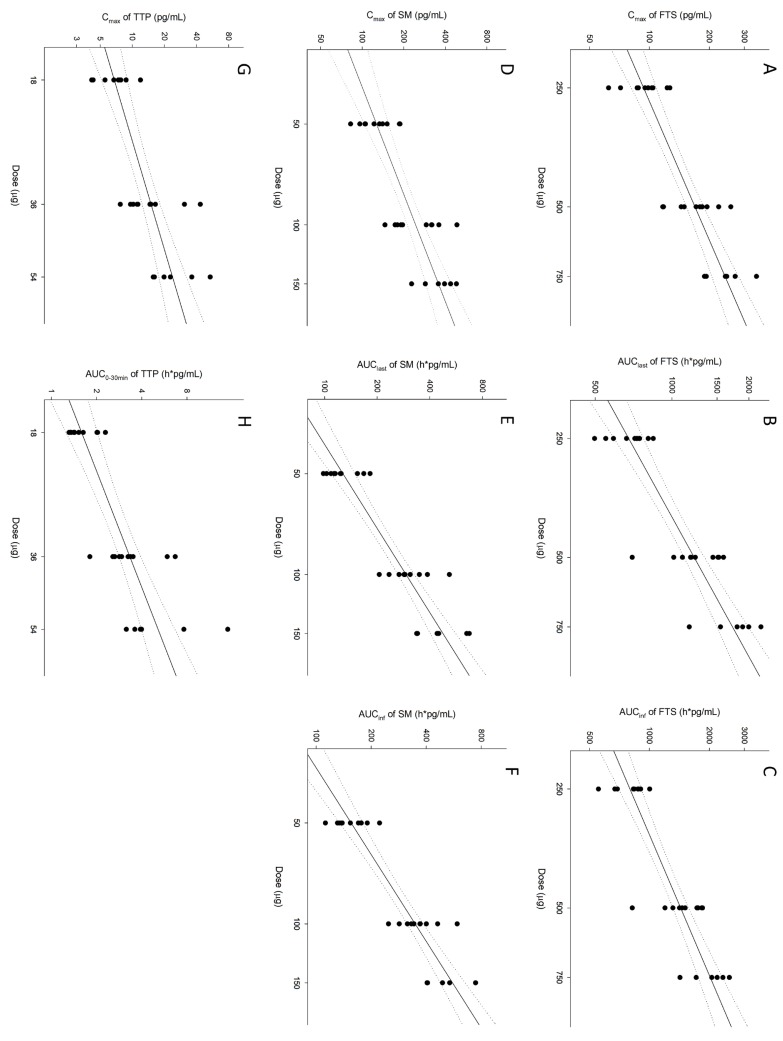
- Chronic obstructive pulmonary disease (COPD) is a type of progressive, obstructive lung disease characterized by long-term poor airflow. The symptoms of COPD may be relieved and its progression delayed by fluticasone (FTS), salmeterol (SM), and tiotropium (TTP). The aim of this study is to investigate pharmacokinetic (PK) characteristics of inhaled FTS, SM, and TTP after co-administration. An open-label, single-arm, three-period, simple ascending dose study was conducted in 10 healthy male subjects. A single dose of FTS/SM (250/50 µg) and TTP (18 µg) were concomitantly inhaled in period 1, and the dose of each drug was escalated to two- and three-fold in periods 2 and 3, respectively, with a 2-week washout between periods. Activated charcoal was co-administered before and after inhalation to block gastrointestinal absorption. Blood samples for PK analysis were collected up to 24 hours. PK parameters were obtained by non-compartmental analysis. FTS, SM, and TTP rapidly reached maximum plasma concentration after inhalation (0.08-3.00 h, 0.03-0.10 h and 0.03-0.10 h, respectively) and were eliminated with mean half-lives of 9.29-10.44 h, 6.09-12.39 h and 0.25-47.42 h, respectively. PK assessment of the lowest dose of TTP was limited due to relatively low systemic exposure compared to the lower limit of quantification. In conclusion, PK characteristics of FTS, SM, and TTP by pulmonary absorption were evaluated after concurrent inhalation. FTS and SM showed dose-proportional PK profiles between 250-750 µg and 50-150 µg, respectively, while TTP presented dose-proportionality in the early phase exposure between 18-54 µg.
Keyword
MeSH Terms
Figure
Reference
-
1. Koumis T, Samuel S. Tiotropium bromide: A new long-acting bronchodilator for the treatment of chronic obstructive pulmonary disease. Clin Ther. 2005; 27:377–392. DOI: 10.1016/j.clinthera.2005.04.006. PMID: 15922812.
Article2. Cazzola M, Testi R, Matera MG. Clinical pharmacokinetics of salmeterol. Clin Pharmacokinet. 2002; 41:19–30.
Article3. Jung KS, Park HY, Park SY, Kim SK, Kim YK, Shim JJ, et al. Comparison of titotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respir Med. 2012; 106:382–389. DOI: 10.1016/j.rmed.2011.09.004. PMID: 21975275.4. Borgström L, Nilsson M. A method for determination of the absolute pulmonary bioavailability of inhaled drugs: terbutaline. Pharm Res. 1990; 7:1068–1070. PMID: 2281038.5. Gough K, Hutchison M, Keene O, Byrom B, Ellis S, Lacey L, et al. Assessment of dose proportionality: Report from the statisticians in the pharmaceutical industry/pharmacokinetics UK joint working party. Ther Innov Regul Sci. 1995; 29:1039–1048.
Article6. Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000; 17:1278–1283. PMID: 11145235.7. Daley-Yates PT, Mehta R, Chan RH, Despa SX, Louey MD. Pharmacokinetics and pharmacodynamics of fluticasone propionate and salmeterol delivered as a combination dry powder from a capsule-based inhaler and a multidose inhaler in asthma and COPD patients. J Aerosol Med Pulm Drug Deliv. 2014; 27:279–289. DOI: 10.1089/jamp.2013.1040. PMID: 24074143.
Article8. Mehta R, Montembault M, Warren F, Gupta A, Brealey N, Moore A. Systemic exposures of fluticasone propionate and salmeterol following inhalation via metered dose inhaler with the mini spacer compared with aerochamber plus spacer. J Aerosol Med Pulm Drug Deliv. 2016; 29:386–392. DOI: 10.1089/jamp.2015.1236. PMID: 26824933.9. Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007; 146:545–555. PMID: 17310045.10. Dentener MA, Vernooy JH, Hendriks S, Wouters EF. Enhanced levels of hyaluronan in lungs of patients with COPD: relationship with lung function and local inflammation. Thorax. 2005; 60:114–119. PMID: 15681498.
Article11. Meijer RJ, Kerstjens HA, Arends LR, Kauffman HF, Koëter GH, Postma DS. Effects of inhaled fluticasone and oral prednisolone on clinical and inflammatory parameters in patients with asthma. Thorax. 1999; 54:894–899. PMID: 10491451.
Article12. Hochhaus G, Möllmann H, Derendorf H, Gonzalez-Rothi RJ. Pharmacokinetic/Pharmacodynamic aspects of aerosol therapy using glucocorticoids as a model. J Clin Pharmacol. 1997; 37:881–892. PMID: 9505979.
Article13. Disse B, Speck GA, Rominger KL, Witek TJ Jr, Hammer R. Tiotropium (Spiriva ™): Mechanistical considerations and clinical profile in obstructive lung disease. Life Sci. 1999; 64:457–464. PMID: 10069510.14. Durham MC. Tiotropium (Spiriva): a once-daily inhaled anticholinergic medication for chronic obstructive pulmonary disease. Proc (Bayl Univ Med Cent). 2004; 17:366–373. PMID: 16200123.
Article15. Brutsche MH, Brutsche IC, Munawar M, Langley SJ, Masterson CM, Daley-Yates PT, et al. Comparison of pharmacokinetics and systemic effects of inhaled fluticasone propionate in patients with asthma and healthy volunteers: a randomized crossover study. Lancet. 2000; 356:556–561. PMID: 10950233.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison for the Effects of Triple Therapy with Salmeterol/Fluticasone Propionate and Tiotropium Bromide versus Individual Components in Patients of Severe COPD Combined with Bronchial Hyperresponsiveness
- A Comparison of Salmeterol with Salbutamol Inhalation in Treatment of Mild to Moderate Asthma
- Clinical studies of salmeterol
- The role of tiotropium in the management of asthma
- Add-on Tiotropium in Chinese Patients With Moderate Asthma: A Pooled Subgroup Analysis of MezzoTinA-Asthma 1 and 2