J Pathol Transl Med.
2015 Nov;49(6):472-480. 10.4132/jptm.2015.09.11.
Therapeutic Effects of Umbilical Cord Blood Derived Mesenchymal Stem Cell-Conditioned Medium on Pulmonary Arterial Hypertension in Rats
- Affiliations
-
- 1Department of Biology, School of Life Sciences, Chungbuk National University, Cheongju, Korea. leejc@chungbuk.ac.kr
- 2Department of Surgery, Brain Korea 21 PLUS Project for Medical Sciences and HBP Surgery and Liver Transplantation, Korea University College of Medicine, Seoul, Korea. beas100@korea.ac.kr
- 3Department of Anatomy, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2381406
- DOI: http://doi.org/10.4132/jptm.2015.09.11
Abstract
- BACKGROUND
Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) may have multiple therapeutic applications for cell based therapy including the treatment of pulmonary artery hypertension (PAH). As low survival rates and potential tumorigenicity of implanted cells could undermine the mesenchymal stem cell (MSC) cell-based therapy, we chose to investigate the use of conditioned medium (CM) from a culture of MSC cells as a feasible alternative.
METHODS
CM was prepared by culturing hUCB-MSCs in three-dimensional spheroids. In a rat model of PAH induced by monocrotaline, we infused CM or the control unconditioned culture media via the tail-vein of 6-week-old Sprague-Dawley rats.
RESULTS
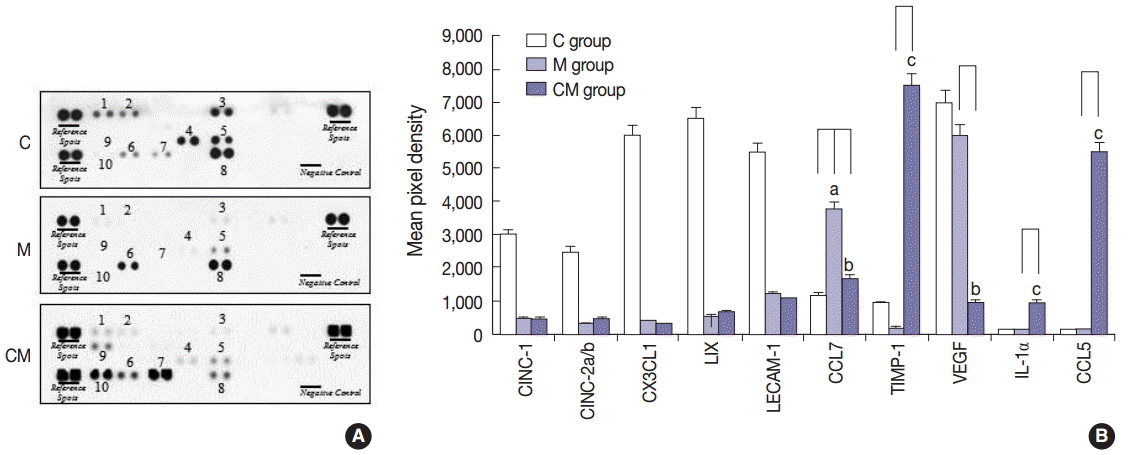
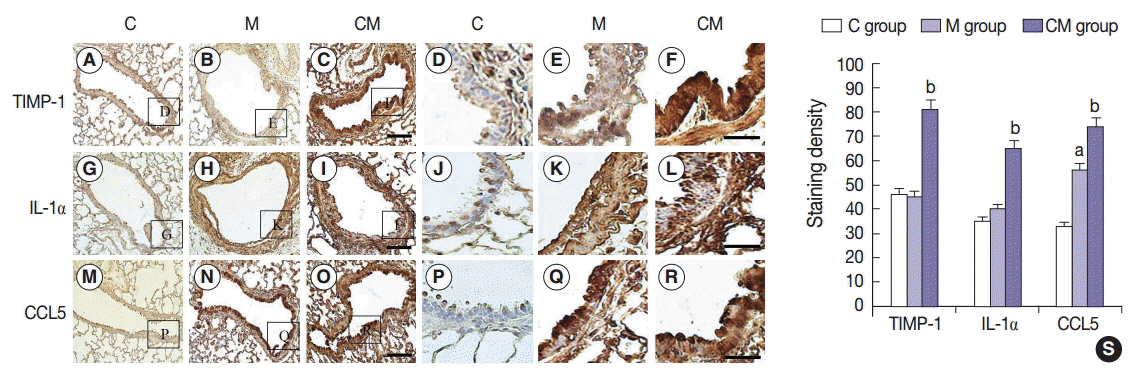
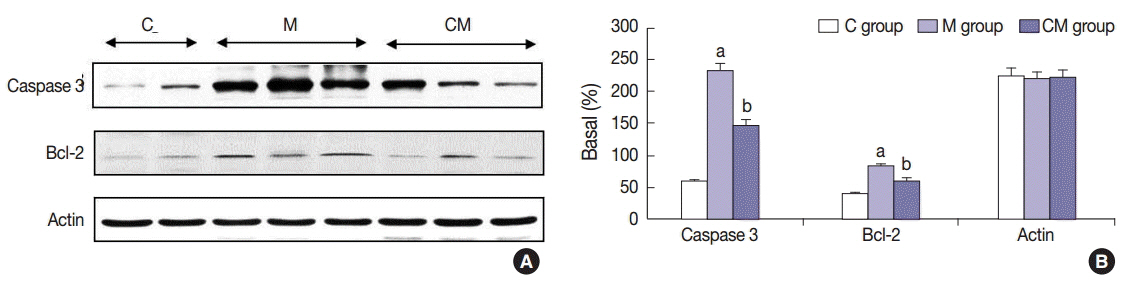
Compared with the control unconditioned media, CM infusion reduced the ventricular pressure, the right ventricle/(left ventricle+interventricular septum) ratio, and maintained respiratory function in the treated animals. Also, the number of interleukin 1alpha (IL-1alpha), chemokine (C-C motif) ligand 5 (CCL5), and tissue inhibitor of metalloproteinase 1 (TIMP-1)-positive cells increased in lung samples and the number of terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling technique (TUNEL)-positive cells decreased significantly in the CM treated animals.
CONCLUSIONS
From our in vivo data in the rat model, the observed decreases in the TUNEL staining suggest a potential therapeutic benefit of the CM in ameliorating PAH-mediated lung tissue damage. Increased IL-1alpha, CCL5, and TIMP-1 levels may play important roles in this regard.
Keyword
MeSH Terms
-
Animals
Apoptosis
Culture Media
Culture Media, Conditioned
Deoxyuridine
Fetal Blood*
Gene Expression
Humans
Hypertension*
In Situ Nick-End Labeling
Interleukin-1alpha
Lung
Mesenchymal Stromal Cells
Models, Animal
Monocrotaline
Pulmonary Artery
Rats*
Rats, Sprague-Dawley
Survival Rate
Tissue Inhibitor of Metalloproteinase-1
Umbilical Cord*
Ventricular Pressure
Culture Media
Culture Media, Conditioned
Deoxyuridine
Interleukin-1alpha
Monocrotaline
Tissue Inhibitor of Metalloproteinase-1
Figure
Cited by 1 articles
-
Notice of Retraction: Therapeutic Effects of Umbilical Cord Blood Derived Mesenchymal Stem Cell-Conditioned Medium on Pulmonary Arterial Hypertension in Rats
Jae Chul Lee, Choong Ik Cha, Dong-Sik Kim, Soo Young Choe
J Pathol Transl Med. 2016;50(4):325-325. doi: 10.4132/jptm.2015.09.11.r.
Reference
-
1. Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004; 351:1655–65.
Article2. Liang OD, Mitsialis SA, Chang MS, et al. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011; 29:99–107.
Article3. Can MM, Tanboga IH, Demircan HC, et al. Enhanced hemostatic indices in patients with pulmonary arterial hypertension: an observational study. Thromb Res. 2010; 126:280–2.
Article4. Fukumoto Y, Shimokawa H. Recent progress in the management of pulmonary hypertension. Circ J. 2011; 75:1801–10.
Article5. Liu Q, Luo Z, He S, et al. Conditioned serum-free medium from umbilical cord mesenchymal stem cells has anti-photoaging properties. Biotechnol Lett. 2013; 35:1707–14.
Article6. Lee JC, Kim KC, Yang YS, et al. Microarray analysis after umbilical cord blood derived mesenchymal stem cells injection in monocrotaline-induced pulmonary artery hypertension rats. Anat Cell Biol. 2014; 47:217–26.
Article7. Zhao YD, Courtman DW, Ng DS, et al. Microvascular regeneration in established pulmonary hypertension by angiogenic gene transfer. Am J Respir Cell Mol Biol. 2006; 35:182–9.
Article8. Saigawa T, Kato K, Ozawa T, et al. Clinical application of bone marrow implantation in patients with arteriosclerosis obliterans, and the association between efficacy and the number of implanted bone marrow cells. Circ J. 2004; 68:1189–93.
Article9. Kang H, Kim KH, Lim J, et al. The therapeutic effects of human mesenchymal stem cells primed with sphingosine-1 phosphate on pulmonary artery hypertension. Stem Cells Dev. 2015; 24:1658–71.
Article10. Umar S, de Visser YP, Steendijk P, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009; 297:H1606–16.
Article11. Kim AK, Kim MH, Kim S, et al. Stem-cell therapy for peripheral arterial occlusive disease. Eur J Vasc Endovasc Surg. 2011; 42:667–75.
Article12. Lee KB, Kang ES, Kim AK, et al. Stem cell therapy in patients with thromboangiitis obliterans: assessment of the long-term clinical outcome and analysis of the prognostic factors. Int J Stem Cells. 2011; 4:88–98.
Article13. Yang SS, Kim NR, Park KB, et al. A phase I study of human cord blood-derived mesenchymal stem cell therapy in patients with peripheral arterial occlusive disease. Int J Stem Cells. 2013; 6:37–44.
Article14. Cho YJ, Song HS, Bhang S, et al. Therapeutic effects of human adipose stem cell-conditioned medium on stroke. J Neurosci Res. 2012; 90:1794–802.
Article15. Lim HC, Lee ST, Chu K, et al. Neuroprotective effect of neural stem cell-conditioned media in in vitro model of Huntington’s disease. Neurosci Lett. 2008; 435:175–80.
Article16. Lee EJ, Park SJ, Kang SK, et al. Spherical bullet formation via E-cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Mol Ther. 2012; 20:1424–33.
Article17. Pereira T, Ivanova G, Caseiro AR, et al. MSCs conditioned media and umbilical cord blood plasma metabolomics and composition. PLoS One. 2014; 9:e113769.
Article18. Lipke DW, Arcot SS, Gillespie MN, Olson JW. Temporal alterations in specific basement membrane components in lungs from monocrotaline-treated rats. Am J Respir Cell Mol Biol. 1993; 9:418–28.
Article19. Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004; 22:625–34.
Article20. Wang M, Yang Y, Yang D, et al. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009; 126:220–32.21. Jeong SY, Kim DH, Ha J, et al. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013; 31:2136–48.
Article22. Kim JY, Kim DH, Kim JH, et al. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-beta plaques. Cell Death Differ. 2012; 19:680–91.23. Jin H, Sanberg PR, Henning RJ. Human umbilical cord blood mononuclear cell-conditioned media inhibits hypoxic-induced apoptosis in human coronary artery endothelial cells and cardiac myocytes by activation of the survival protein Akt. Cell Transplant. 2013; 22:1637–50.
Article24. Yang S, Sun HM, Yan JH, et al. Conditioned medium from human amniotic epithelial cells may induce the differentiation of human umbilical cord blood mesenchymal stem cells into dopaminergic neuron-like cells. J Neurosci Res. 2013; 91:978–86.
Article25. Yang C, Lei D, Ouyang W, et al. Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitro. Biomed Res Int. 2014; 2014:109389.
Article26. Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002; 418:41–9.
Article27. Mareschi K, Ferrero I, Rustichelli D, et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006; 97:744–54.
Article28. Secco M, Zucconi E, Vieira NM, et al. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008; 26:146–50.
Article29. Kim ES, Jeon HB, Lim H, et al. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells inhibits melanogenesis by promoting proteasomal degradation of MITF. PLoS One. 2015; 10:e0128078.
Article30. Kim JY, Kim DH, Kim DS, et al. Galectin-3 secreted by human umbilical cord blood-derived mesenchymal stem cells reduces amyloid-beta42 neurotoxicity in vitro. FEBS Lett. 2010; 584:3601–8.
Article31. Flynn A, Barry F, O’Brien T. UC blood-derived mesenchymal stromal cells: an overview. Cytotherapy. 2007; 9:717–26.
Article32. Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004; 4:11–22.
Article33. Chandrasekar B, Melby PC, Sarau HM, et al. Chemokine-cytokine cross-talk. The ELR+ CXC chemokine LIX (CXCL5) amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase-NF-kappa B pathway. J Biol Chem. 2003; 278:4675–86.34. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000; 1477:267–83.
Article35. Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010; 20:161–8.
Article36. Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002; 21:2245–52.
Article37. Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008; 1:re6.
Article38. Furie MB, Randolph GJ. Chemokines and tissue injury. Am J Pathol. 1995; 146:1287–301.39. Kaneider NC, Leger AJ, Kuliopulos A. Therapeutic targeting of molecules involved in leukocyte-endothelial cell interactions. FEBS J. 2006; 273:4416–24.
Article40. Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001; 107:1529–36.41. Johnston CJ, Piedboeuf B, Rubin P, Williams JP, Baggs R, Finkelstein JN. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res. 1996; 145:762–7.42. Phan SH, Kunkel SL. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992; 18:29–43.
Article43. Pan LH, Ohtani H, Yamauchi K, Nagura H. Co-expression of TNF alpha and IL-1 beta in human acute pulmonary fibrotic diseases: an immunohistochemical analysis. Pathol Int. 1996; 46:91–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Notice of Retraction: Therapeutic Effects of Umbilical Cord Blood Derived Mesenchymal Stem Cell-Conditioned Medium on Pulmonary Arterial Hypertension in Rats
- Efficacy of a Hair Tonic Containing Human Umbilical Cord Blood Mesenchymal Stem Cell-derived Conditioned Media in Patients with Androgenetic Alopecia
- Effect of Conditioned Medium from Human Umbilical Cord-Derived Mesenchymal Stromal Cells on Rejuvenation of Nucleus Pulposus Derived Stem/Progenitor Cells from Degenerated Intervertebral Disc
- Microarray analysis after umbilical cord blood derived mesenchymal stem cells injection in monocrotaline-induced pulmonary artery hypertension rats
- Endothelial progenitor cells and mesenchymal stem cells from human cord blood