J Korean Med Sci.
2017 Jul;32(7):1139-1146. 10.3346/jkms.2017.32.7.1139.
Helicobacter pylori Antigens Inducing Early Immune Response in Infants
- Affiliations
-
- 1Department of Pediatrics, Gyeongsang National Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Korea. hsyoun@gnu.ac.kr
- 2Department of Pathology, Gyeongsang National Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Korea.
- 3Department of Microbiology, Gyeongsang National Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Korea.
- KMID: 2379609
- DOI: http://doi.org/10.3346/jkms.2017.32.7.1139
Abstract
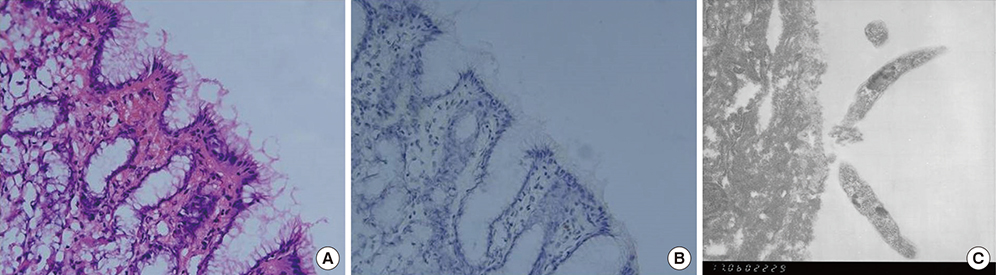
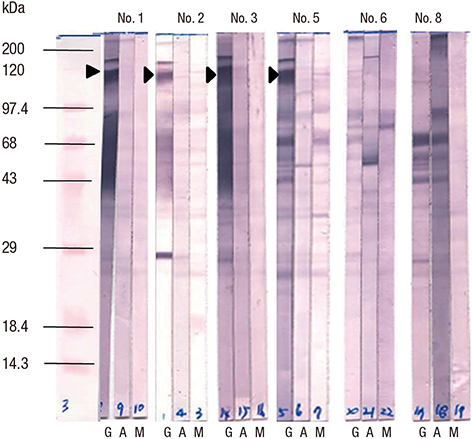
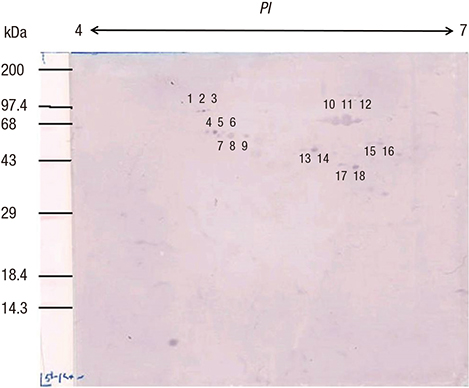
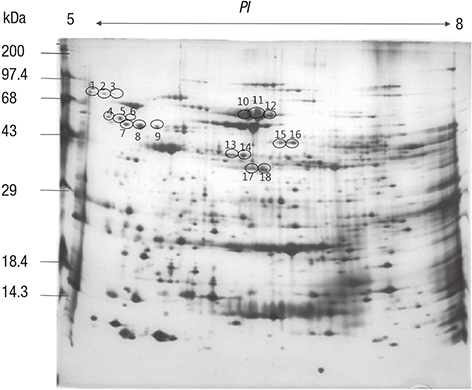
- To identify the Helicobacter pylori antigens operating during early infection in sera from infected infants using proteomics and immunoblot analysis. Two-dimensional (2D) large and small gel electrophoresis was performed using H. pylori strain 51. We performed 2D immunoglobulin G (IgG), immunoglobulin A (IgA), and immunoglobulin M (IgM) antibody immunoblotting using small gels on sera collected at the Gyeongsang National University Hospital from 4-11-month-old infants confirmed with H. pylori infection by pre-embedding immunoelectron microscopy. Immunoblot spots appearing to represent early infection markers in infant sera were compared to those of the large 2D gel for H. pylori strain 51. Corresponding spots were analyzed by matrix-assisted laser desorption/ionization time of flight-mass spectrometry (MALDI-TOF-MS). The peptide fingerprints obtained were searched in the National Center for Biotechnology Information (NCBI) database. Eight infant patients were confirmed with H. pylori infection based on urease tests, histopathologic examinations, and pre-embedding immunoelectron microscopy. One infant showed a 2D IgM immunoblot pattern that seemed to represent early infection. Immunoblot spots were compared with those from whole-cell extracts of H. pylori strain 51 and 18 spots were excised, digested in gel, and analyzed by MALDI-TOF-MS. Of the 10 peptide fingerprints obtained, the H. pylori proteins flagellin A (FlaA), urease β subunit (UreB), pyruvate ferredoxin oxidoreductase (POR), and translation elongation factor Ts (EF-Ts) were identified and appeared to be active during the early infection periods. These results might aid identification of serological markers for the serodiagnosis of early H. pylori infection in infants.
MeSH Terms
-
Biotechnology
Electrophoresis
Flagellin
Gels
Helicobacter pylori*
Helicobacter*
Humans
Immunoblotting
Immunoglobulin A
Immunoglobulin G
Immunoglobulin M
Infant*
Microscopy, Immunoelectron
Peptide Elongation Factors
Peptide Mapping
Proteomics
Pyruvate Synthase
Serologic Tests
Spectrum Analysis
Urease
Flagellin
Gels
Immunoglobulin A
Immunoglobulin G
Immunoglobulin M
Peptide Elongation Factors
Pyruvate Synthase
Urease
Figure
Reference
-
1. Blaser MJ. Helicobacter pylori and gastric diseases. BMJ. 1998; 316:1507–1510.2. Rhee KH, Youn HS, Baik SC, Lee WK, Cho MJ, Choi HJ, Maeng KY, Ko KW. Prevalence of Helicobacter pylori infection in Korea. J Korean Soc Microbiol. 1990; 25:475–490.3. Gologan A, Graham DY, Sepulveda AR. Molecular markers in Helicobacter pylori-associated gastric carcinogenesis. Clin Lab Med. 2005; 25:197–222.4. Seo JH, Park JS, Yeom JS, Lim JY, Park CH, Woo HO, Baik SC, Lee WK, Cho MJ, Rhee KH, et al. Correlation between positive rate and number of biopsy samples on urease test in childhood Helicobacter pylori infection. J Korean Med Sci. 2014; 29:106–109.5. Crabtree JE, Mahony MJ, Taylor JD, Heatley RV, Littlewood JM, Tompkins DS. Immune responses to Helicobacter pylori in children with recurrent abdominal pain. J Clin Pathol. 1991; 44:768–771.6. Kindermann A, Konstantopoulos N, Lehn N, Demmelmair H, Koletzko S. Evaluation of two commercial enzyme immunoassays, testing immunoglobulin G (IgG) and IgA responses, for diagnosis of Helicobacter pylori infection in children. J Clin Microbiol. 2001; 39:3591–3596.7. Oleastro M, Matos R, Cabral J, Barros R, Lopes AI, Ramalho P, Monteiro L. Evaluation of a Western blot test, Helico Blot 2.1, in the diagnosis of Helicobacter pylori infection in a pediatric population. Helicobacter. 2002; 7:210–215.8. Akada J, Okuda M, Hiramoto N, Kitagawa T, Zhang X, Kamei S, Ito A, Nakamura M, Uchida T, Hiwatani T, et al. Proteomic characterization of Helicobacter pylori CagA antigen recognized by child serum antibodies and its epitope mapping by peptide array. PLoS One. 2014; 9:e104611.9. Seo JH, Lim CW, Park JS, Yeom JS, Lim JY, Jun JS, Woo HO, Youn HS, Baik SC, Lee WK, et al. Correlations between the CagA antigen and serum levels of anti-Helicobacter pylori IgG and IgA in children. J Korean Med Sci. 2016; 31:417–422.10. Haas G, Karaali G, Ebermayer K, Metzger WG, Lamer S, Zimny-Arndt U, Diescher S, Goebel UB, Vogt K, Roznowski AB, et al. Immunoproteomics of Helicobacter pylori infection and relation to gastric disease. Proteomics. 2002; 2:313–324.11. Jung HS, Kim EJ, Kim EA, Park JH, Jun JS, Seo JH, Lim JY, Choi MB, Woo HO, Youn HS, et al. Detection of Helicobacter pylori by pre-embedding immunoelectron microscopy: comparison with immunoblotting method. J Korean Pediatr Soc. 2002; 45:862–874.12. Cho MJ, Jeon BS, Park JW, Jung TS, Song JY, Lee WK, Choi YJ, Choi SH, Park SG, Park JU, et al. Identifying the major proteome components of Helicobacter pylori strain 26695. Electrophoresis. 2002; 23:1161–1173.13. Jung TS, Kang SC, Choi YJ, Jeon BS, Park JW, Jung SA, Song JY, Choi SH, Park SG, Choe MY, et al. Two-dimensional gel electrophoresis of Helicobacter pylori for proteomic analysis. J Korean Soc Microbiol. 2000; 35:97–108.14. O’Connell KL, Stults JT. Identification of mouse liver proteins on two-dimensional electrophoresis gels by matrix-assisted laser desorption/ionization mass spectrometry of in situ enzymatic digests. Electrophoresis. 1997; 18:349–359.15. Lin YF, Chen CY, Tsai MH, Wu MS, Wang YC, Chuang EY, Lin JT, Yang PC, Chow LP. Duodenal ulcer-related antigens from Helicobacter pylori: immunoproteome and protein microarray approaches. Mol Cell Proteomics. 2007; 6:1018–1026.16. Lin YF, Wu MS, Chang CC, Lin SW, Lin JT, Sun YJ, Chen DS, Chow LP. Comparative immunoproteomics of identification and characterization of virulence factors from Helicobacter pylori related to gastric cancer. Mol Cell Proteomics. 2006; 5:1484–1496.17. Mitchell HM, Hazell SL, Kolesnikow T, Mitchell J, Frommer D. Antigen recognition during progression from acute to chronic infection with a cagA-positive strain of Helicobacter pylori . Infect Immun. 1996; 64:1166–1172.18. Kim EA, Kim YO, Lim JY, Jung YS, Park CH, Woo HO, Youn HS, Ko GH, Baik SC, Lee WK, et al. Antibody response of infants to Helicobacter pylori infection. Korean J Gastroenterol. 2000; 35:704–715.19. Lock RA, Coombs GW, McWilliams TM, Pearman JW, Grubb WB, Melrose GJ, Forbes GM. Proteome analysis of highly immunoreactive proteins of Helicobacter pylori . Helicobacter. 2002; 7:175–182.20. Suerbaum S, Josenhans C, Labigne A. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993; 175:3278–3288.21. Labigne A, Cussac V, Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991; 173:1920–1931.22. Narikawa S, Imai N, Yamamoto M, Suzuki T, Yanagawa A, Mizushima Y. Oxygen and carbon dioxide requirements of Helicobacter pylori . Acta Microbiol Immunol Hung. 1995; 42:367–371.23. Schmitt L, Tampé R. Structure and mechanism of ABC transporters. Curr Opin Struct Biol. 2002; 12:754–760.24. Wang D, Luo B, Shan W, Hao M, Sun X, Ge R. The effects of EF-Ts and bismuth on EF-Tu in Helicobacter pylori: implications for an elegant timing for the introduction of EF-Ts in the elongation and EF-Tu as a potential drug target. Metallomics. 2013; 5:888–895.25. Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999; 397:176–180.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antibody Response of Infants to Helicobacter pylori Infection
- Immune Response to Helicobacter pylori Infection
- Response to Treatment of Helicobacter pylori-associated Dyspepsia: Eradication of Helicobacter pylori or Correction of Gastric or Intestinal Dysbiosis?
- A Case of Immune Thrombocytopenic Purpura with Helicobacter Pylori Infection
- Helicobacter pylori Infection and Extra-gastroduodenal Diseases