Obstet Gynecol Sci.
2016 Nov;59(6):470-478. 10.5468/ogs.2016.59.6.470.
Glycogen synthase kinase 3β and cyclin D1 expression in cervical carcinogenesis
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea.
- 2Department of Pathology, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea. hojunglee@eulji.ac.kr
- KMID: 2378571
- DOI: http://doi.org/10.5468/ogs.2016.59.6.470
Abstract
OBJECTIVE
Glycogen synthase kinase 3β (GSK3β) is a pluripotent protein kinase involved in the development of cancers through regulation of numerous oncogenic molecules. Cyclin D1, an important regulator of G1 to S phase transition in various cells, is one of target proteins that GSK3β regulate. Our objective was to assess the expression of GSK3β and cyclin D1 in cervical neoplasm of different histologic grades and to identify their correlation in cervical carcinogenesis.
METHODS
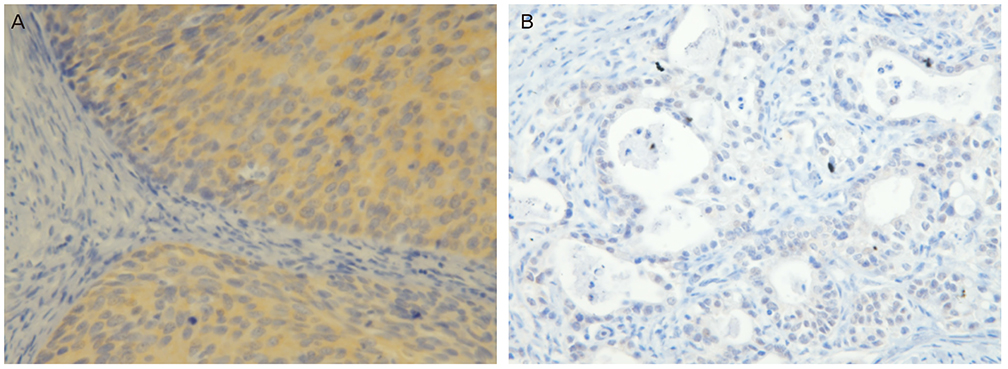
Immunohistochemical analysis of GSK3β and cyclin D1 was performed in a total of 137 patients with 12 normal, 62 cervical intraepithelial neoplasia (CIN) (31 CIN1 and 31 CIN3) and 63 invasive cancers including 56 squamous cell carcinomas and 7 adenocarcinomas.
RESULTS
The expression of GSK3β increased in parallel with the lesion grade, while that of cyclin D1 decreased with severity of the lesion (P<0.001). There was a significant inverse correlation between GSK3β and cyclin D1 expression in overall cervical neoplasia (Φ=-0.413, P<0.001). GSK3β expression was higher in squamous cell carcinoma than in adenocarcinoma (P=0.049).
CONCLUSION
These results suggest that the expressional increase in GSK3β plays a role in cervical carcinogenesis and has inverse correlation with cyclin D1 expression in this process. In addition, GSK3β expression appears to be associated with the histologic type of cervical cancer, especially squamous cell carcinoma.
Keyword
MeSH Terms
-
Adenocarcinoma
Carcinogenesis*
Carcinoma, Squamous Cell
Cervical Intraepithelial Neoplasia
Cyclin D1*
Cyclins*
Glycogen Synthase Kinases*
Glycogen Synthase*
Glycogen*
Humans
Immunohistochemistry
Protein Kinases
S Phase
Uterine Cervical Neoplasms
Cyclin D1
Cyclins
Glycogen
Glycogen Synthase
Glycogen Synthase Kinases
Protein Kinases
Figure
Cited by 1 articles
-
Different expression of GSK3β and pS9GSK3β depending on phenotype of cervical cancer: possible association of GSK3β with squamous cell carcinoma and pS9GSK3β with adenocarcinoma
Kwanghee Ahn, Sojung Kweon, Dae Woon Kim, Hojung Lee
Obstet Gynecol Sci. 2019;62(3):157-165. doi: 10.5468/ogs.2019.62.3.157.
Reference
-
1. HPV Information Centre. Human papillomavirus and related diseases report [Internet]. Barcelona: ICO Information Centre on HPV and Cancer;2016. 2016 Jan 28. Available from: http://www.hpvcentre.net/statistics/reports/XWX.pdf.2. zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000; 92:690–698.3. Bahnassy AA, Zekri AR, Saleh M, Lotayef M, Moneir M, Shawki O. The possible role of cell cycle regulators in multistep process of HPV-associated cervical carcinoma. BMC Clin Pathol. 2007; 7:4.4. Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond). 2006; 110:525–541.5. Howley PM, Scheffner M, Huibregtse J, Munger K. Oncoproteins encoded by the cancer-associated human papillomaviruses target the products of the retinoblastoma and p53 tumor suppressor genes. Cold Spring Harb Symp Quant Biol. 1991; 56:149–155.6. Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010; 10:550–560.7. Perez-Plasencia C, Vazquez-Ortiz G, Lopez-Romero R, Pina-Sanchez P, Moreno J, Salcedo M. Genome wide expression analysis in HPV16 cervical cancer: identification of altered metabolic pathways. Infect Agent Cancer. 2007; 2:16.8. Vandermark ER, Deluca KA, Gardner CR, Marker DF, Schreiner CN, Strickland DA, et al. Human papillomavirus type 16 E6 and E 7 proteins alter NF-kB in cultured cervical epithelial cells and inhibition of NF-kB promotes cell growth and immortalization. Virology. 2012; 425:53–60.9. Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003; 116(Pt 7):1175–1186.10. Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004; 29:95–102.11. McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Sokolosky M, Abrams SL, et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 2014; 5:2881–2911.12. Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000; 14:2501–2514.13. Tullai JW, Chen J, Schaffer ME, Kamenetsky E, Kasif S, Cooper GM. Glycogen synthase kinase-3 represses cyclic AMP response element-binding protein (CREB)-targeted immediate early genes in quiescent cells. J Biol Chem. 2007; 282:9482–9491.14. Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998; 12:3499–3511.15. Ougolkov AV, Fernandez-Zapico ME, Bilim VN, Smyrk TC, Chari ST, Billadeau DD. Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clin Cancer Res. 2006; 12:5074–5081.16. Karrasch T, Spaeth T, Allard B, Jobin C. PI3K-dependent GSK3ß(Ser9)-phosphorylation is implicated in the intestinal epithelial cell wound-healing response. PLoS One. 2011; 6:e26340.17. Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010; 9:144.18. Mishra R, Nagini S, Rana A. Expression and inactivation of glycogen synthase kinase 3 alpha/beta and their association with the expression of cyclin D1 and p53 in oral squamous cell carcinoma progression. Mol Cancer. 2015; 14:20.19. Shakoori A, Mai W, Miyashita K, Yasumoto K, Takahashi Y, Ooi A, et al. Inhibition of GSK-3 beta activity attenuates proliferation of human colon cancer cells in rodents. Cancer Sci. 2007; 98:1388–1393.20. Naito S, Bilim V, Yuuki K, Ugolkov A, Motoyama T, Nagaoka A, et al. Glycogen synthase kinase-3beta: a prognostic marker and a potential therapeutic target in human bladder cancer. Clin Cancer Res. 2010; 16:5124–5132.21. Cao Q, Lu X, Feng YJ. Glycogen synthase kinase-3beta positively regulates the proliferation of human ovarian cancer cells. Cell Res. 2006; 16:671–677.22. Bilim V, Ougolkov A, Yuuki K, Naito S, Kawazoe H, Muto A, et al. Glycogen synthase kinase-3: a new therapeutic target in renal cell carcinoma. Br J Cancer. 2009; 101:2005–2014.23. Miyashita K, Kawakami K, Nakada M, Mai W, Shakoori A, Fujisawa H, et al. Potential therapeutic effect of glycogen synthase kinase 3beta inhibition against human glioblastoma. Clin Cancer Res. 2009; 15:887–897.24. Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993; 7:331–342.25. Leone G, DeGregori J, Jakoi L, Cook JG, Nevins JR. Collaborative role of E2F transcriptional activity and G1 cyclindependent kinase activity in the induction of S phase. Proc Natl Acad Sci U S A. 1999; 96:6626–6631.26. Bartkova J, Lukas J, Muller H, Strauss M, Gusterson B, Bartek J. Abnormal patterns of D-type cyclin expression and G1 regulation in human head and neck cancer. Cancer Res. 1995; 55:949–956.27. Cheung TH, Yu MM, Lo KW, Yim SF, Chung TK, Wong YF. Alteration of cyclin D1 and CDK4 gene in carcinoma of uterine cervix. Cancer Lett. 2001; 166:199–206.28. Ma C, Wang J, Gao Y, Gao TW, Chen G, Bower KA, et al. The role of glycogen synthase kinase 3beta in the transformation of epidermal cells. Cancer Res. 2007; 67:7756–7764.29. Skomedal H, Kristensen GB, Lie AK, Holm R. Aberrant expression of the cell cycle associated proteins TP53, MDM2, p21, p27, cdk4, cyclin D1, RB, and EGFR in cervical carcinomas. Gynecol Oncol. 1999; 73:223–228.30. Cho NH, Kim YT, Kim JW. Correlation between G1 cyclins and HPV in the uterine cervix. Int J Gynecol Pathol. 1997; 16:339–347.31. Bae DS, Cho SB, Kim YJ, Whang JD, Song SY, Park CS, et al. Aberrant expression of cyclin D1 is associated with poor prognosis in early stage cervical cancer of the uterus. Gynecol Oncol. 2001; 81:341–347.32. Rath G, Jawanjal P, Salhan S, Nalliah M, Dhawan I. Clinical significance of inactivated glycogen synthase kinase 3β in HPV-associated cervical cancer: Relationship with Wnt/β-catenin pathway activation. Am J Reprod Immunol. 2015; 73:460–478.33. Choi SK, Hong YO, Lee WM, Kim EK, Joo JE, Kim DW, et al. Overexpression of PI3K-p110α in the progression of uterine cervical neoplasia and its correlation with pAkt and DJ-1. Eur J Gynaecol Oncol. 2015; 36:389–393.34. de Groot RP, Auwerx J, Bourouis M, Sassone-Corsi P. Negative regulation of Jun/AP-1: conserved function of glycogen synthase kinase 3 and the Drosophila kinase shaggy. Oncogene. 1993; 8:841–847.35. Manoukian AS, Woodgett JR. Role of glycogen synthase kinase-3 in cancer: regulation by Wnts and other signaling pathways. Adv Cancer Res. 2002; 84:203–229.36. Nichols GE, Williams ME, Gaffey MJ, Stoler MH. Cyclin D1 gene expression in human cervical neoplasia. Mod Pathol. 1996; 9:418–425.37. Lukas J, Muller H, Bartkova J, Spitkovsky D, Kjerulff AA, Jansen-Durr P, et al. DNA tumor virus oncoproteins and retinoblastoma gene mutations share the ability to relieve the cell’s requirement for cyclin D1 function in G1. J Cell Biol. 1994; 125:625–638.38. Koay MH, Crook M, Stewart CJ. Cyclin D1, E-cadherin and beta-catenin expression in FIGO Stage IA cervical squamous carcinoma: diagnostic value and evidence for epithelial-mesenchymal transition. Histopathology. 2012; 61:1125–1133.39. Casimiro MC, Crosariol M, Loro E, Li Z, Pestell RG. Cyclins and cell cycle control in cancer and disease. Genes Cancer. 2012; 3:649–657.40. Li Z, Wang C, Prendergast GC, Pestell RG. Cyclin D1 functions in cell migration. Cell Cycle. 2006; 5:2440–2442.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phosphorylation of glycogen synthase kinase-3beta at serine-9 by phospholipase Cgamma1 through protein kinase C in rat 3Y1 fibroblasts
- Expression of Cyclin D1 and CDK4 in DMBA-Induced Rat Ovarian Cancer
- Suppression of β-catenin Signaling Pathway in Human Prostate Cancer PC3 Cells by Delphinidin
- Cyclin B1 and D1 expression in invasive cervical cancer
- Lithium ameliorates rat spinal cord injury by suppressing glycogen synthase kinase-3β and activating heme oxygenase-1