Anat Cell Biol.
2017 Sep;50(3):207-213. 10.5115/acb.2017.50.3.207.
Lithium ameliorates rat spinal cord injury by suppressing glycogen synthase kinase-3β and activating heme oxygenase-1
- Affiliations
-
- 1Department of Veterinary Anatomy, College of Veterinary Medicine, Jeju National University, Jeju, Korea. shint@jejunu.ac.kr, healthy@jejunu.ac.kr
- 2HAEMALGEUN Veterinary Medical Clinic, Jeju, Korea.
- KMID: 2390482
- DOI: http://doi.org/10.5115/acb.2017.50.3.207
Abstract
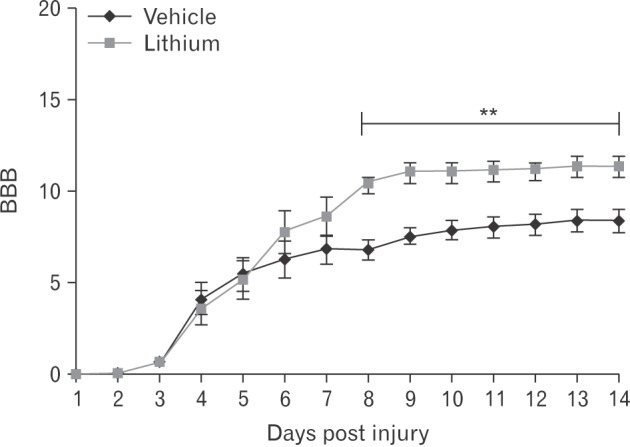
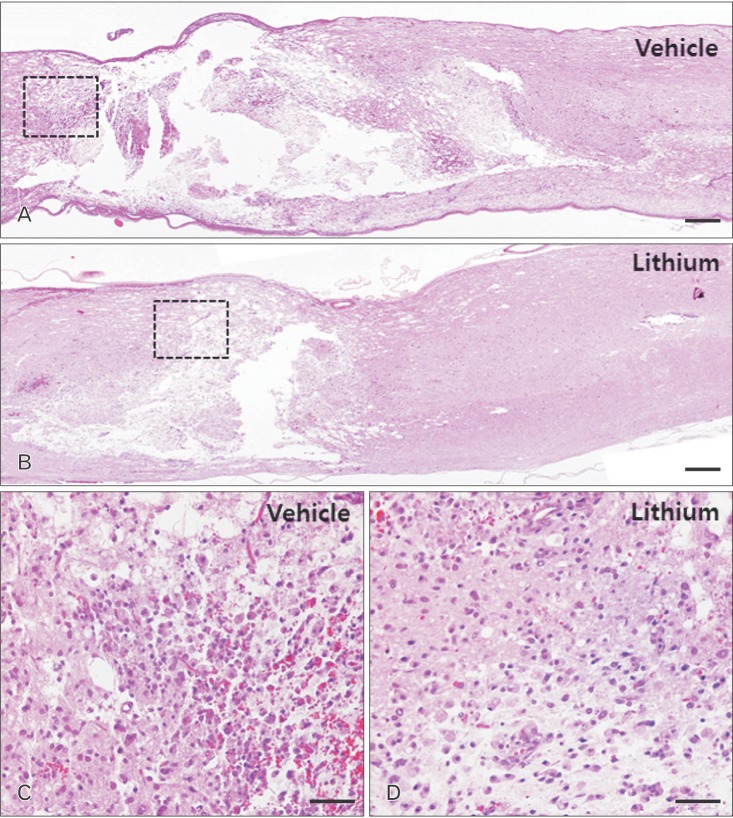
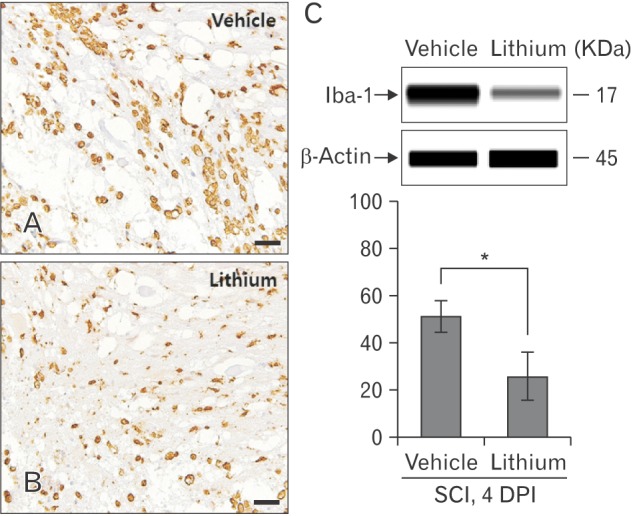
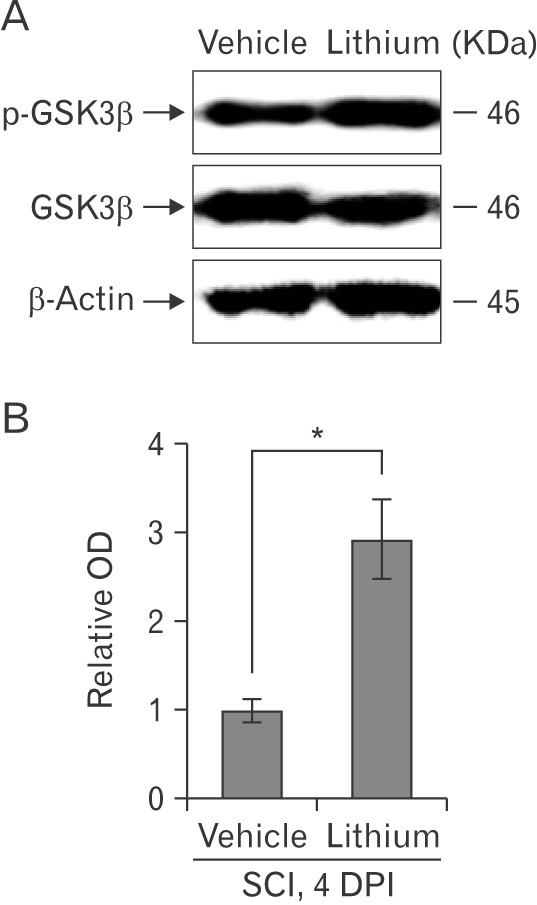
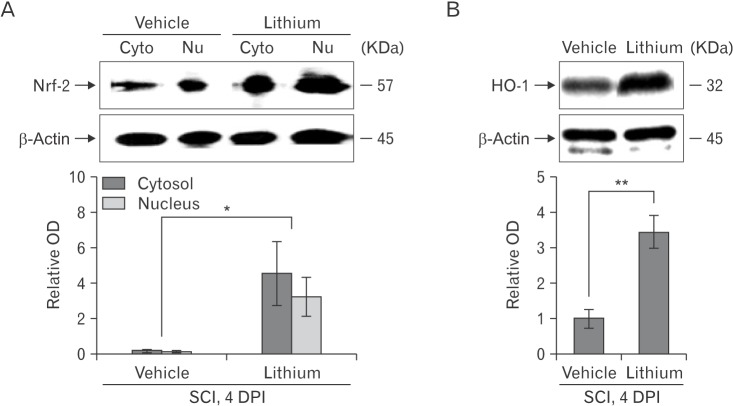
- Glycogen synthase kinase (GSK)-3β and related enzymes are associated with various forms of neuroinflammation, including spinal cord injury (SCI). Our aim was to evaluate whether lithium, a non-selective inhibitor of GSK-3β, ameliorated SCI progression, and also to analyze whether lithium affected the expression levels of two representative GSK-3β-associated molecules, nuclear factor erythroid 2-related factor-2 (Nrf-2) and heme oxygenase-1 (HO-1) (a target gene of Nrf-2). Intraperitoneal lithium chloride (80 mg/kg/day for 3 days) significantly improved locomotor function at 8 days post-injury (DPI); this was maintained until 14 DPI (P<0.05). Western blotting showed significantly increased phosphorylation of GSK-3β (Ser9), Nrf-2, and the Nrf-2 target HO-1 in the spinal cords of lithium-treated animals. Fewer neuropathological changes (e.g., hemorrhage, inflammatory cell infiltration, and tissue loss) were observed in the spinal cords of the lithium-treated group compared with the vehicle-treated group. Microglial activation (evaluated by measuring the immunoreactivity of ionized calcium-binding protein-1) was also significantly reduced in the lithium-treated group. These findings suggest that GSK-3β becomes activated after SCI, and that a non-specific enzyme inhibitor, lithium, ameliorates rat SCI by increasing phosphorylation of GSK-3β and the associated molecules Nrf-2 and HO-1.
Keyword
MeSH Terms
Figure
Reference
-
1. Jung K, Min DS, Sim KB, Ahn M, Kim H, Cheong J, Shin T. Upregulation of phospholipase D1 in the spinal cords of rats with clip compression injury. Neurosci Lett. 2003; 336:126–130. PMID: 12499056.2. Ahn M, Moon C, Park C, Kim J, Sim KB, Shin T. Transient activation of an adaptor protein, disabled-2, in rat spinal cord injury. Acta Histochem. 2015; 117:56–61. PMID: 25432322.3. Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004; 4:451–464. PMID: 15246307.4. Shin T, Ahn M, Moon C, Kim S, Sim KB. Alternatively activated macrophages in spinal cord injury and remission: another mechanism for repair? Mol Neurobiol. 2013; 47:1011–1019. PMID: 23321790.5. Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008; 209:378–388. PMID: 17662717.6. Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001; 65:391–426. PMID: 11527574.7. De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002; 43:1158–1164. PMID: 12504922.8. Ahn M, Kim J, Park C, Cho J, Jee Y, Jung K, Moon C, Shin T. Potential involvement of glycogen synthase kinase (GSK)-3beta in a rat model of multiple sclerosis: evidenced by lithium treatment. Anat Cell Biol. 2017; 50:48–59. PMID: 28417055.9. Fang XY, Zhang WM, Zhang CF, Wong WM, Li W, Wu W, Lin JH. Lithium accelerates functional motor recovery by improving remyelination of regenerating axons following ventral root avulsion and reimplantation. Neuroscience. 2016; 329:213–225. PMID: 27185485.10. Young W. Review of lithium effects on brain and blood. Cell Transplant. 2009; 18:951–975. PMID: 19523343.11. Pratheeshkumar P, Son YO, Divya SP, Roy RV, Hitron JA, Wang L, Kim D, Dai J, Asha P, Zhang Z, Wang Y, Shi X. Luteolin inhibits Cr(VI)-induced malignant cell transformation of human lung epithelial cells by targeting ROS mediated multiple cell signaling pathways. Toxicol Appl Pharmacol. 2014; 281:230–241. PMID: 25448439.12. Jiang W, Li M, He F, Bian Z, He Q, Wang X, Yao W, Zhu L. Neuroprotective effect of asiatic acid against spinal cord injury in rats. Life Sci. 2016; 157:45–51. PMID: 27153777.13. Ahn M, Kim J, Bang H, Moon J, Kim GO, Shin T. Hepatoprotective effects of allyl isothiocyanate against carbon tetrachloride-induced hepatotoxicity in rat. Chem Biol Interact. 2016; 254:102–108. PMID: 27241356.14. Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, Kunsch C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006; 290:H1862–H1870. PMID: 16339837.15. Lv R, Mao N, Wu J, Lu C, Ding M, Gu X, Wu Y, Shi Z. Neuroprotective effect of allicin in a rat model of acute spinal cord injury. Life Sci. 2015; 143:114–123. PMID: 26546416.16. Kim DH, Heo SD, Ahn MJ, Sim KB, Shin TK. Activation of embryonic intermediate filaments contributes to glial scar formation after spinal cord injury in rats. J Vet Sci. 2003; 4:109–112. PMID: 14610361.17. Ahn M, Lee C, Jung K, Kim H, Moon C, Sim KB, Shin T. Immunohistochemical study of arginase-1 in the spinal cords of rats with clip compression injury. Brain Res. 2012; 1445:11–19. PMID: 22325098.18. Koshinaga M, Whittemore SR. The temporal and spatial activation of microglia in fiber tracts undergoing anterograde and retrograde degeneration following spinal cord lesion. J Neurotrauma. 1995; 12:209–222. PMID: 7629867.19. Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994; 6:712–724. PMID: 8075816.20. Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010; 7:366–377. PMID: 20880501.21. Kim H, Moon C, Ahn M, Byun J, Lee Y, Kim MD, Matsumoto Y, Koh CS, Shin T. Heat shock protein 27 upregulation and phosphorylation in rat experimental autoimmune encephalomyelitis. Brain Res. 2009; 1304:155–163. PMID: 19781527.22. Chen JQ, Heldman MR, Herrmann MA, Kedei N, Woo W, Blumberg PM, Goldsmith PK. Absolute quantitation of endogenous proteins with precision and accuracy using a capillary Western system. Anal Biochem. 2013; 442:97–103. PMID: 23896461.23. Vijayaprakash KM, Sridharan N. An experimental spinal cord injury rat model using customized impact device: a cost-effective approach. J Pharmacol Pharmacother. 2013; 4:211–213. PMID: 23960429.24. Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006; 23:635–659. PMID: 16689667.25. Shang AJ, Yang Y, Wang HY, Tao BZ, Wang J, Wang ZF, Zhou DB. Spinal cord injury effectively ameliorated by neuroprotective effects of rosmarinic acid. Nutr Neurosci. 2017; 20:172–179. PMID: 26796989.26. Wang ZH, Xie YX, Zhang JW, Qiu XH, Cheng AB, Tian L, Ma BY, Hou YB. Carnosol protects against spinal cord injury through Nrf-2 upregulation. J Recept Signal Transduct Res. 2016; 36:72–78. PMID: 26791582.27. Kesherwani V, Atif F, Yousuf S, Agrawal SK. Resveratrol protects spinal cord dorsal column from hypoxic injury by activating Nrf-2. Neuroscience. 2013; 241:80–88. PMID: 23523995.28. Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011; 31:1121–1133. PMID: 21245377.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Potential involvement of glycogen synthase kinase (GSK)-3β in a rat model of multiple sclerosis: evidenced by lithium treatment
- The Anti-Inflammatory Activity of Eucommia ulmoides Oliv. Bark. Involves NF-κB Suppression and Nrf2-Dependent HO-1 Induction in BV-2 Microglial Cells
- Lithium alleviates paralysis in experimental autoimmune neuritis in Lewis rats by modulating glycogen synthase kinase-3β activity
- Wnt and GSK3 Signaling Pathways in Bipolar Disorder: Clinical and Therapeutic Implications
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases