Anat Cell Biol.
2017 Mar;50(1):48-59. 10.5115/acb.2017.50.1.48.
Potential involvement of glycogen synthase kinase (GSK)-3β in a rat model of multiple sclerosis: evidenced by lithium treatment
- Affiliations
-
- 1Department of Veterinary Anatomy, College of Veterinary Medicine, Jeju National University, Jeju, Korea. shint@jejunu.ac.kr
- 2Department of Molecular Anatomy, School of Medicine, University of the Ryukyus, Nishihara, Japan.
- 3Eco-friendly Material Research Center, Korea Research Institute of Bioscience and Biotechnology, Jeongeup, Korea.
- 4Department of Veterinary Anatomy, College of Veterinary Medicine, Chonnam National University, Gwangju, Korea.
- KMID: 2390426
- DOI: http://doi.org/10.5115/acb.2017.50.1.48
Abstract
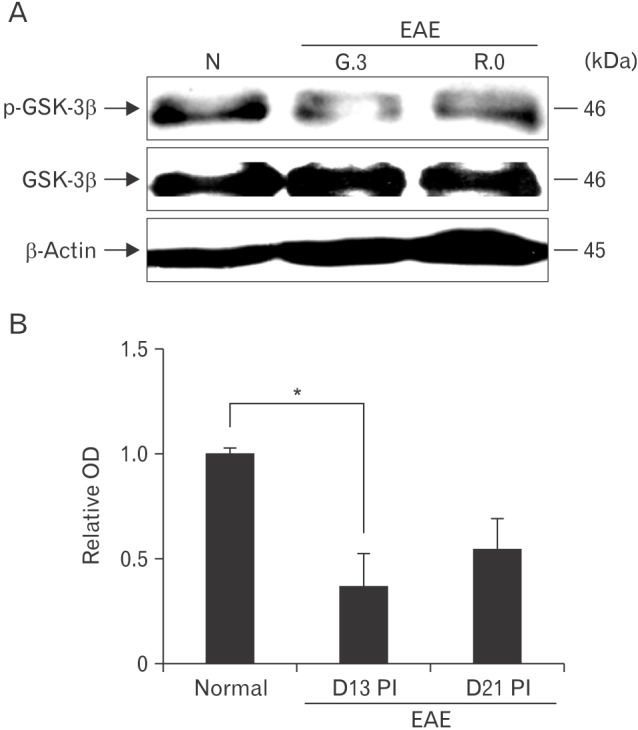
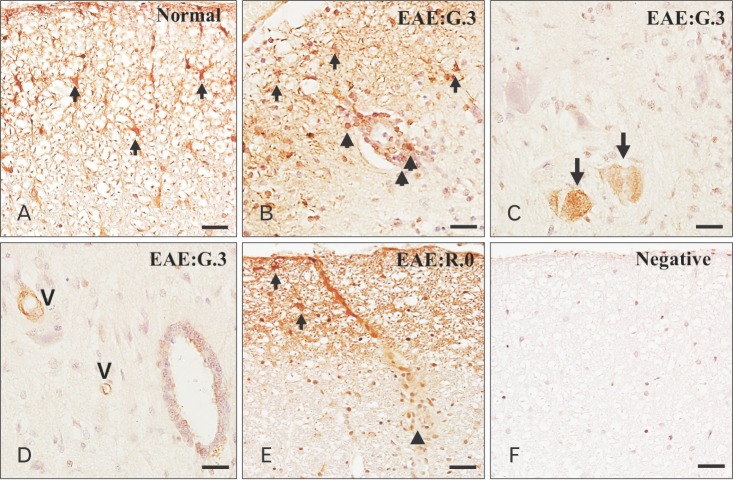
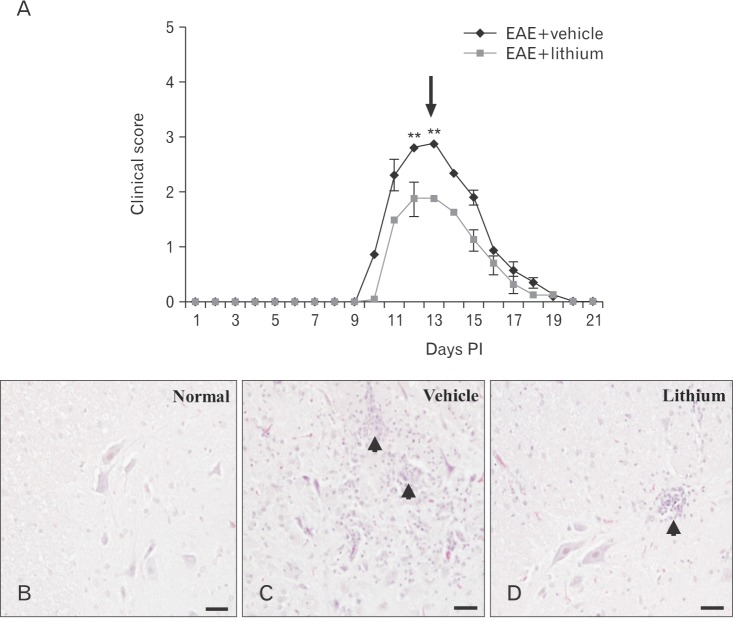
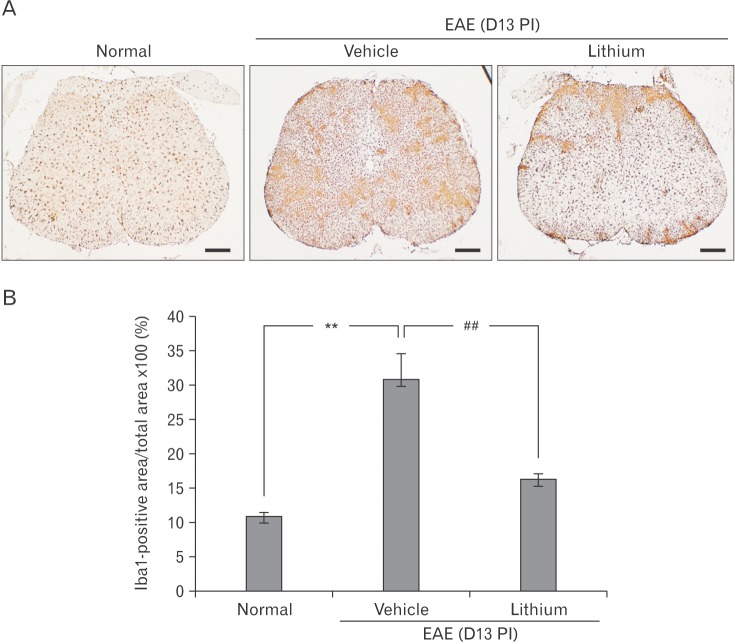
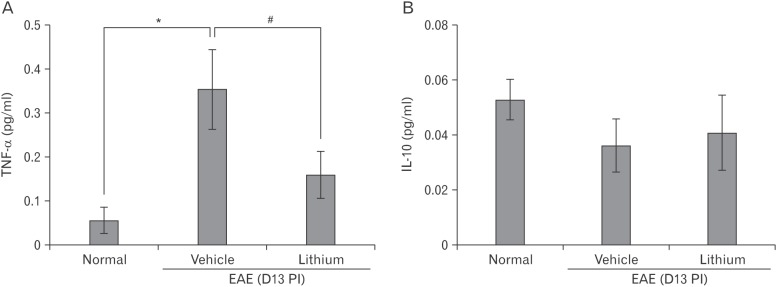
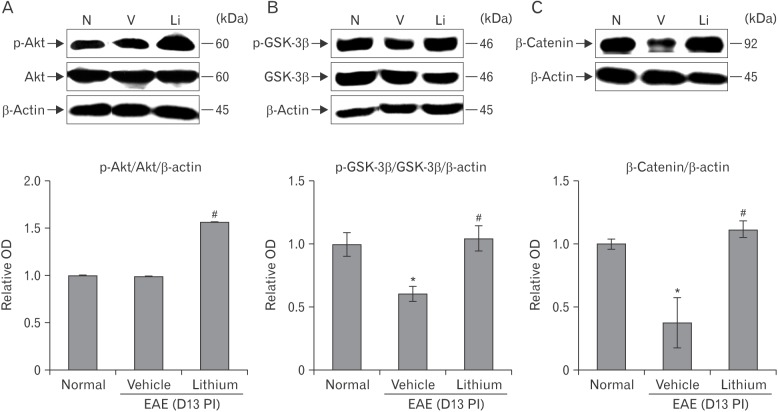
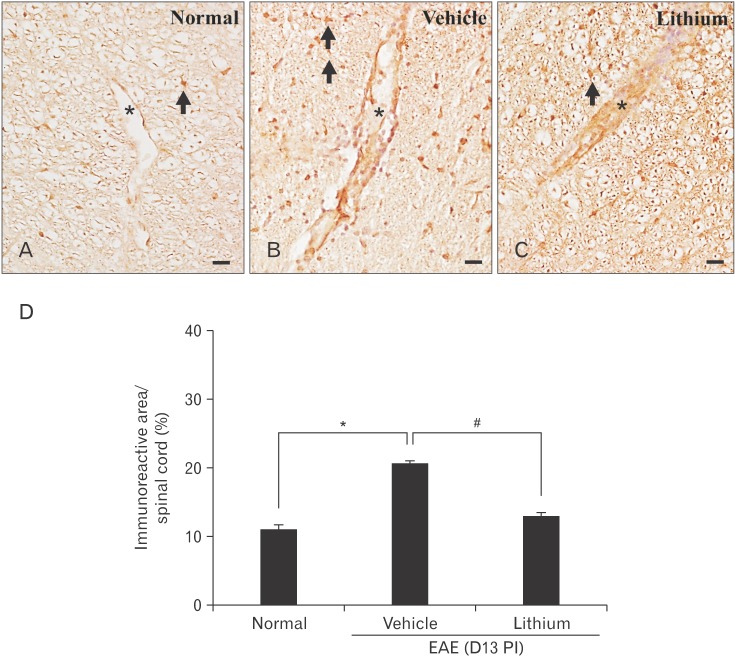
- Glycogen synthase kinase (GSK)-3β has been known as a pro-inflammatory molecule in neuroinflammation. The involvement of GSK-3β remains unsolved in acute monophasic rat experimental autoimmune encephalomyelitis (EAE). The aim of this study was to evaluate a potential role of GSK-3β in central nervous system (CNS) autoimmunity through its inhibition by lithium. Lithium treatment significantly delayed the onset of EAE paralysis and ameliorated its severity. Lithium treatment reduced the serum level of pro-inflammatory tumor necrosis factor a but not that of interleukin 10. Western blot analysis showed that the phosphorylation of GSK-3β (p-GSK-3β) and its upstream factor Akt was significantly increased in the lithium-treated group. Immunohistochemical examination revealed that lithium treatment also suppressed the activation of ionized calcium binding protein-1-positive microglial cells and vascular cell adhesion molecule-1 expression in the spinal cords of lithium-treated EAE rats. These results demonstrate that lithium ameliorates clinical symptom of acute monophasic rat EAE, and GSK-3 is a target for the suppression of acute neuroinflammation as far as rat model of human CNS disease is involved.
MeSH Terms
-
Animals
Autoimmunity
Blotting, Western
Calcium
Central Nervous System
Central Nervous System Diseases
Encephalomyelitis, Autoimmune, Experimental
Glycogen Synthase Kinase 3
Glycogen Synthase Kinases*
Glycogen Synthase*
Glycogen*
Humans
Interleukin-10
Lithium*
Models, Animal*
Multiple Sclerosis*
Paralysis
Phosphorylation
Rats*
Spinal Cord
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Calcium
Glycogen
Glycogen Synthase
Glycogen Synthase Kinase 3
Glycogen Synthase Kinases
Interleukin-10
Lithium
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Figure
Reference
-
1. Shin T, Ahn M, Matsumoto Y. Mechanism of experimental autoimmune encephalomyelitis in Lewis rats: recent insights from macrophages. Anat Cell Biol. 2012; 45:141–148. PMID: 23094201.2. Shin T, Kojima T, Tanuma N, Ishihara Y, Matsumoto Y. The subarachnoid space as a site for precursor T cell proliferation and effector T cell selection in experimental autoimmune encephalomyelitis. J Neuroimmunol. 1995; 56:171–178. PMID: 7860712.3. Swanborg RH. Experimental autoimmune encephalomyelitis in the rat: lessons in T-cell immunology and autoreactivity. Immunol Rev. 2001; 184:129–135. PMID: 12086308.4. Matsumoto Y. New approach to immunotherapy against organ-specific autoimmune diseases with T cell receptor and chemokine receptor DNA vaccines. Curr Drug Targets Immune Endocr Metabol Disord. 2005; 5:73–77. PMID: 15777206.5. Ahn M, Yang W, Kim H, Jin JK, Moon C, Shin T. Immunohistochemical study of arginase-1 in the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis. Brain Res. 2012; 1453:77–86. PMID: 22483960.6. Ellrichmann G, Thöne J, Lee DH, Rupec RA, Gold R, Linker RA. Constitutive activity of NF-kappa B in myeloid cells drives pathogenicity of monocytes and macrophages during autoimmune neuroinflammation. J Neuroinflammation. 2012; 9:15. PMID: 22260436.7. Kim H, Moon C, Ahn M, Lee Y, Kim S, Matsumoto Y, Koh CS, Kim MD, Shin T. Increased phosphorylation of cyclic AMP response element-binding protein in the spinal cord of Lewis rats with experimental autoimmune encephalomyelitis. Brain Res. 2007; 1162:113–120. PMID: 17617386.8. Aleshin S, Strokin M, Sergeeva M, Reiser G. Peroxisome proliferator-activated receptor (PPAR)beta/delta, a possible nexus of PPARalpha- and PPARgamma-dependent molecular pathways in neurodegenerative diseases: Review and novel hypotheses. Neurochem Int. 2013; 63:322–330. PMID: 23811400.9. Chen G, Shannon M. Transcription factors and th17 cell development in experimental autoimmune encephalomyelitis. Crit Rev Immunol. 2013; 33:165–182. PMID: 23582061.10. Beurel E. Regulation by glycogen synthase kinase-3 of inflammation and T cells in CNS diseases. Front Mol Neurosci. 2011; 4:18. PMID: 21941466.11. Beurel E, Kaidanovich-Beilin O, Yeh WI, Song L, Palomo V, Michalek SM, Woodgett JR, Harrington LE, Eldar-Finkelman H, Martinez A, Jope RS. Regulation of Th1 cells and experimental autoimmune encephalomyelitis by glycogen synthase kinase-3. J Immunol. 2013; 190:5000–5011. PMID: 23606540.12. Rowse AL, Naves R, Cashman KS, McGuire DJ, Mbana T, Raman C, De Sarno P. Lithium controls central nervous system autoimmunity through modulation of IFN-gamma signaling. PLoS One. 2012; 7:e52658. PMID: 23285134.13. De Sarno P, Axtell RC, Raman C, Roth KA, Alessi DR, Jope RS. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2008; 181:338–345. PMID: 18566399.14. Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001; 65:391–426. PMID: 11527574.15. Eto M, Kouroedov A, Cosentino F, Lüscher TF. Glycogen synthase kinase-3 mediates endothelial cell activation by tumor necrosis factor-alpha. Circulation. 2005; 112:1316–1322. PMID: 16129813.16. Ramirez SH, Fan S, Zhang M, Papugani A, Reichenbach N, Dykstra H, Mercer AJ, Tuma RF, Persidsky Y. Inhibition of glycogen synthase kinase 3beta (GSK3beta) decreases inflammatory responses in brain endothelial cells. Am J Pathol. 2010; 176:881–892. PMID: 20056834.17. Lee MJ, Jang M, Choi J, Chang BS, Kim DY, Kim SH, Kwak YS, Oh S, Lee JH, Chang BJ, Nah SY, Cho IH. Korean red ginseng and ginsenoside-Rb1/-Rg1 alleviate experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating regulatory T cells. Mol Neurobiol. 2016; 53:1977–2002. PMID: 25846819.18. Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci. 2003; 24:441–443. PMID: 12967765.19. Levine S, Saltzman A. Inhibition of experimental allergic encephalomyelitis by lithium chloride: specific effect or nonspecific stress? Immunopharmacology. 1991; 22:207–213. PMID: 1663498.20. Kim S, Moon C, Wie MB, Kim H, Tanuma N, Matsumoto Y, Shin T. Enhanced expression of constitutive and inducible forms of nitric oxide synthase in autoimmune encephalomyelitis. J Vet Sci. 2000; 1:11–17. PMID: 14612615.21. Kim MD, Cho HJ, Shin T. Expression of osteopontin and its ligand, CD44, in the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004; 151:78–84. PMID: 15145606.22. De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002; 43:1158–1164. PMID: 12504922.23. Kim H, Moon C, Ahn M, Byun J, Lee Y, Kim MD, Matsumoto Y, Koh CS, Shin T. Heat shock protein 27 upregulation and phosphorylation in rat experimental autoimmune encephalomyelitis. Brain Res. 2009; 1304:155–163. PMID: 19781527.24. Dill J, Wang H, Zhou F, Li S. Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci. 2008; 28:8914–8928. PMID: 18768685.25. Tafreshi AP, Payne N, Sun G, Sylvain A, Schulze K, Bernard C. Inactive GSK3beta is disturbed in the spinal cord during experimental autoimmune encephalomyelitis, but rescued by stem cell therapy. Neuroscience. 2014; 277:498–505. PMID: 25064057.26. Weng HR, Gao M, Maixner DW. Glycogen synthase kinase 3 beta regulates glial glutamate transporter protein expression in the spinal dorsal horn in rats with neuropathic pain. Exp Neurol. 2014; 252:18–27. PMID: 24275526.27. Li H, Li Q, Du X, Sun Y, Wang X, Kroemer G, Blomgren K, Zhu C. Lithium-mediated long-term neuroprotection in neonatal rat hypoxia-ischemia is associated with antiinflammatory effects and enhanced proliferation and survival of neural stem/progenitor cells. J Cereb Blood Flow Metab. 2011; 31:2106–2115. PMID: 21587270.28. Kang K, Kim YJ, Kim YH, Roh JN, Nam JM, Kim PY, Ryu WS, Lee SH, Yoon BW. Lithium pretreatment reduces brain injury after intracerebral hemorrhage in rats. Neurol Res. 2012; 34:447–454. PMID: 22450252.29. Dong H, Zhang X, Dai X, Lu S, Gui B, Jin W, Zhang S, Zhang S, Qian Y. Lithium ameliorates lipopolysaccharide-induced microglial activation via inhibition of toll-like receptor 4 expression by activating the PI3K/Akt/FoxO1 pathway. J Neuroinflammation. 2014; 11:140. PMID: 25115727.30. Doverhag C, Hedtjarn M, Poirier F, Mallard C, Hagberg H, Karlsson A, Sävman K. Galectin-3 contributes to neonatal hypoxic-ischemic brain injury. Neurobiol Dis. 2010; 38:36–46. PMID: 20053377.31. Chanaday NL, Roth GA. Microglia and astrocyte activation in the frontal cortex of rats with experimental autoimmune encephalomyelitis. Neuroscience. 2016; 314:160–169. PMID: 26679600.32. Chen K, Wu Y, Zhu M, Deng Q, Nie X, Li M, Wu M, Huang X. Lithium chloride promotes host resistance against Pseudomonas aeruginosa keratitis. Mol Vis. 2013; 19:1502–1514. PMID: 23878501.33. Liu KJ, Lee YL, Yang YY, Shih NY, Ho CC, Wu YC, Huang TS, Huang MC, Liu HC, Shen WW, Leu SJ. Modulation of the development of human monocyte-derived dendritic cells by lithium chloride. J Cell Physiol. 2011; 226:424–433. PMID: 20672290.34. Valvassori SS, Tonin PT, Varela RB, Carvalho AF, Mariot E, Amboni RT, Bianchini G, Andersen ML, Quevedo J. Lithium modulates the production of peripheral and cerebral cytokines in an animal model of mania induced by dextroamphetamine. Bipolar Disord. 2015; 17:507–517. PMID: 25929806.35. Maixner DW, Weng HR. The role of glycogen synthase kinase 3 beta in neuroinflammation and pain. J Pharm Pharmacol (Los Angel). 2013; 1:001. PMID: 25309941.36. Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007; 32:577–595. PMID: 16944320.37. Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010; 31:24–31. PMID: 19836308.38. Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007; 26:227–239. PMID: 17306568.39. Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multitasking kinase. J Cell Sci. 2003; 116(Pt 7):1175–1186. PMID: 12615961.40. Wang H, Brown J, Martin M. Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine. 2011; 53:130–140. PMID: 21095632.41. Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu Y, Pu H, Li WW, Tang B, Wang Y, Gao Y, Zheng P, Bennett MV, Chen J. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc Natl Acad Sci U S A. 2015; 112:2853–2858. PMID: 25691750.42. Raghavendra PB, Lee E, Parameswaran N. Regulation of macrophage biology by lithium: a new look at an old drug. J Neuroimmune Pharmacol. 2014; 9:277–284. PMID: 24277481.43. Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. 2001; 152:87–96. PMID: 11149923.44. Shin T, Ahn M, Jung K, Heo S, Kim D, Jee Y, Lim YK, Yeo EJ. Activation of mitogen-activated protein kinases in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2003; 140:118–125. PMID: 12864979.45. Archambault AS, Sim J, McCandless EE, Klein RS, Russell JH. Region-specific regulation of inflammation and pathogenesis in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006; 181:122–132. PMID: 17030428.46. Gimenez MA, Sim JE, Russell JH. TNFR1-dependent VCAM-1 expression by astrocytes exposes the CNS to destructive inflammation. J Neuroimmunol. 2004; 151:116–125. PMID: 15145610.47. Su Y, Qadri SM, Cayabyab FS, Wu L, Liu L. Regulation of methylglyoxal-elicited leukocyte recruitment by endothelial SGK1/GSK3 signaling. Biochim Biophys Acta. 2014; 1843:2481–2491. PMID: 25003317.48. Kan QC, Zhu L, Liu N, Zhang GX. Matrine suppresses expression of adhesion molecules and chemokines as a mechanism underlying its therapeutic effect in CNS autoimmunity. Immunol Res. 2013; 56:189–196. PMID: 23549837.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lithium ameliorates rat spinal cord injury by suppressing glycogen synthase kinase-3β and activating heme oxygenase-1
- Lithium alleviates paralysis in experimental autoimmune neuritis in Lewis rats by modulating glycogen synthase kinase-3β activity
- Role of Glycogen Synthase Kinase-3 in Motor Neuronal Cell Death Mechanism of in Vitro Familial ALS Model (G94A, A4V mutant motoneuron)
- Immunohistochemical Study on the Distribution of Glycogen Synthase Kinase (GSK) 3beta in the Central Nervous System of SOD1G93A Transgenic Mice
- PI3K-Akt-Wnt Pathway Is Implicated in Exercise-Induced Improvement of Short-term Memory in Cerebral Palsy Rats