IL-32-induced Inflammatory Cytokines Are Selectively Suppressed by α1-antitrypsin in Mouse Bone Marrow Cells
- Affiliations
-
- 1Laboratory of Cytokine Immunology, Department of Biomedical Science and Technology, Konkuk University, Seoul 05029, Korea. soohyun@konkuk.ac.kr
- 2YbdYbiotech Research Center, Seoul 08589, Korea.
- 3Department of Molecular Science and Technology, Ajou University, Suwon 16499, Korea.
- 4College of Veterinary Medicine, Konkuk University, Seoul 05029, Korea.
- 5Division of Pulmonology, Department of Internal Medicine, School of Medicine, Konkuk University, Seoul 05029, Korea.
- 6Department of Medicine, Pusan Paik Hospital, College of Medicine, Inje University, Busan 47392, Korea.
- 7Korea Food Research Institute, Seongnam 13539, Korea.
- 8Departments of Medicine and Academic Affairs, National Jewish Health, Denver, CO 80206, USA.
- 9Division of Nephrology, Department of Internal Medicine, Jeju National University School of Medicine, Jeju 63243, Korea.
- 10College of Veterinary Medicine and Veterinary Science Research Institute, Konkuk University, Seoul 05029, Korea.
- KMID: 2376869
- DOI: http://doi.org/10.4110/in.2017.17.2.116
Abstract
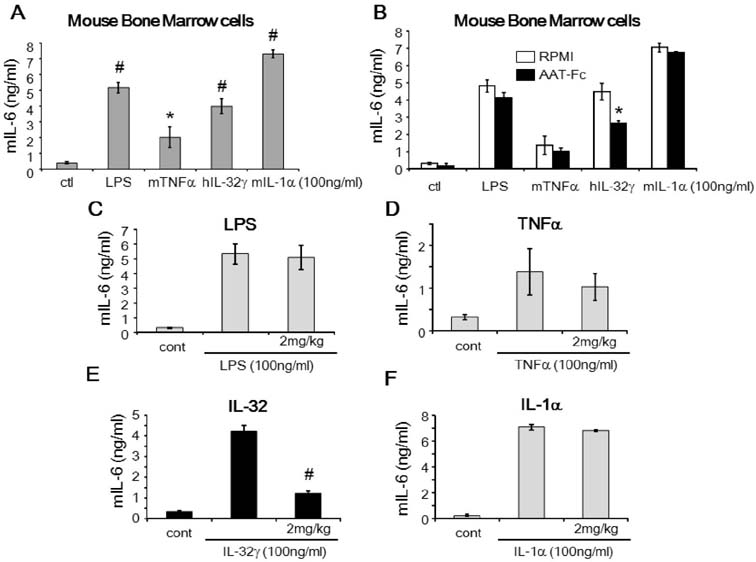
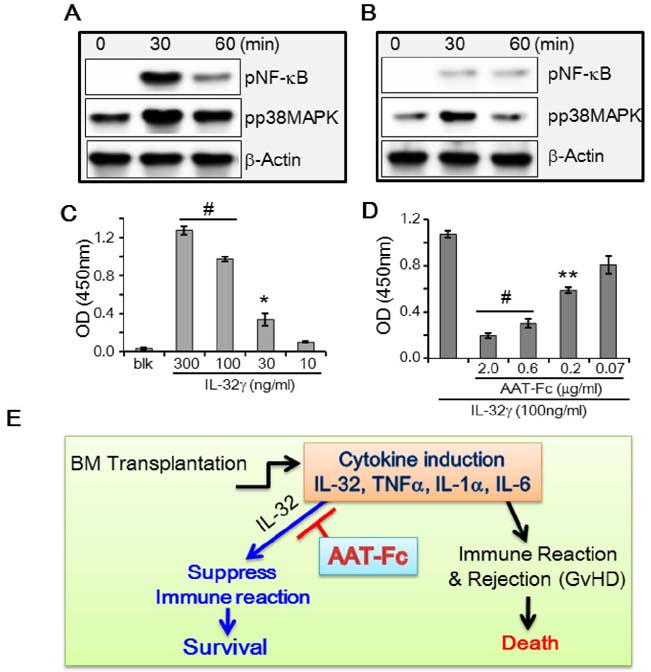
- The induction of interleukin (IL)-32 in bone marrow (BM) inflammation is crucial in graft versus host disease (GvHD) that is a common side effect of allogeneic BM transplantation. Clinical trials on α-1 antitrypsin (AAT) in patients with GvHD are based on the preliminary human and mouse studies on AAT reducing the severity of GvHD. Proteinase 3 (PR3) is an IL-32-binding protein that was isolated from human urine. IL-32 primarily induces inflammatory cytokines in myeloid cells, probably due to PR3 expression on the membrane of the myeloid lineage cells. The inhibitory activity of AAT on serine proteinases may explain the anti-inflammatory effect of AAT on GvHD. However, the anti-inflammatory activity of AAT on BM cells remains unclear. Mouse BM cells were treated with IL-32γ and different inflammatory stimuli to investigate the anti-inflammatory activity of AAT. Recombinant AAT-Fc fusion protein inhibited IL-32γ-induced IL-6 expression in BM cells, but failed to suppress that induced by other stimuli. In addition, the binding of IL-32γ to PR3 was abrogated by AAT-Fc. The data suggest that the specific anti-inflammatory effect of AAT in mouse BM cells is due to the blocking of IL-32 binding to membrane PR3.
MeSH Terms
Figure
Cited by 3 articles
-
Species Specific Antiviral Activity of Porcine Interferon-α8 (IFNα8)
Eunhye Kim, Hyunjhung Jhun, Joohee Kim, Unjoo Park, Seunghyun Jo, Areum Kwak, Sinae Kim, Tam T. Nguyen, Yongsun Kang, Insoo Choi, Joongbok Lee, Heijun Kim, Younghyun Kim, Siyoung Lee, Soohyun Kim
Immune Netw. 2017;17(6):424-436. doi: 10.4110/in.2017.17.6.424.L1 Recombinant Proteins of HPV Tested for Antibody Forming Using Sera of HPV Quadrivalent Vaccine
Begum Akuzum, Sinae Kim, Tam Thanh Nguyen, Jeawoo Hong, Siyoung Lee, Eunhye Kim, Joohee Kim, Yeook Choi, Hyunjhung Jhun, Youngmin Lee, Hyunwoo Kim, Dong Hyun Sohn, Soohyun Kim
Immune Netw. 2018;18(3):. doi: 10.4110/in.2018.18.e19.Structural Characteristics of Seven IL-32 Variants
Dong Hyun Sohn, Tam T. Nguyen, Sinae Kim, Saerok Shim, Siyoung Lee, Youngmin Lee, Hyunjhung Jhun, Tania Azam, Joohee Kim, Soohyun Kim
Immune Netw. 2019;19(2):. doi: 10.4110/in.2019.19.e8.
Reference
-
1. Schwartz RH, Van Ess JD, Johnstone DE, Dreyfuss EM, Abrishami MA, Chai H. Alpha-1 antitrypsin in childhood asthma. J Allergy Clin Immunol. 1977; 59:31–34.
Article2. Becker K, Frieling T, Haussinger D. Quantification of fecal alpha 1-antitrypsin excretion for assessment of inflammatory bowel diseases. Eur J Med Res. 1998; 3:65–70.3. Campbell EJ, Campbell MA, Boukedes SS, Owen CA. Quantum proteolysis by neutrophils: implications for pulmonary emphysema in alpha 1-antitrypsin deficiency. J Clin Invest. 1999; 104:337–344.
Article4. Elzouki AN, Eriksson S, Lofberg R, Nassberger L, Wieslander J, Lindgren S. The prevalence and clinical significance of alpha 1-antitrypsin deficiency (PiZ) and ANCA specificities (proteinase 3, BPI) in patients with ulcerative colitis. Inflamm Bowel Dis. 1999; 5:246–252.
Article5. Malerba M, Radaeli A, Ceriani L, Tantucci C, Grassi V. Airway hyperresponsiveness in a large group of subjects with alpha1-antitrypsin deficiency: a cross-sectional controlled study. J Intern Med. 2003; 253:351–358.
Article6. Aldonyte R, Eriksson S, Piitulainen E, Wallmark A, Janciauskiene S. Analysis of systemic biomarkers in COPD patients. COPD. 2004; 1:155–164.
Article7. Griese M, Latzin P, Kappler M, Weckerle K, Heinzlmaier T, Bernhardt T, Hartl D. alpha1-Antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur Respir J. 2007; 29:240–250.
Article8. Kwak A, Lee Y, Kim H, Kim S. Intracellular interleukin (IL)-1 family cytokine processing enzyme. Arch Pharm Res. 2016; 39:1556–1564.
Article9. Novick D, Rubinstein M, Azam T, Rabinkov A, Dinarello CA, Kim SH. Proteinase 3 is an IL-32 binding protein. Proc Natl Acad Sci U S A. 2006; 103:3316–3321.
Article10. Kim S, Lee S, Her E, Bae S, Choi J, Hong J, Jaekal J, Yoon D, Azam T, Dinarello CA, Kim S. Proteinase 3-processed form of the recombinant IL-32 separate domain. BMB Rep. 2008; 41:814–819.
Article11. Bae S, Kang T, Hong J, Lee S, Choi J, Jhun H, Kwak A, Hong K, Kim E, Jo S, Kim S. Contradictory functions (activation/termination) of neutrophil proteinase 3 enzyme (PR3) in interleukin-33 biological activity. J Biol Chem. 2012; 287:8205–8213.
Article12. Kim SJ, Lee S, Kwak A, Kim E, Jo S, Bae S, Lee Y, Ryoo S, Choi J, Kim S. Interleukin-32gamma transgenic mice resist LPS-mediated septic shock. J Microbiol Biotechnol. 2014; 24:1133–1142.13. Kim S. Interleukin-32 in inflammatory autoimmune diseases. Immune Netw. 2014; 14:123–127.
Article14. Jhun H, Choi J, Hong J, Lee S, Kwak A, Kim E, Jo S, Ryoo S, Lim Y, Yoon DY, Hong JT, Kim TS, Lee Y, Song K, Kim S. IL-32gamma overexpression accelerates streptozotocin (STZ)-induced type 1 diabetes. Cytokine. 2014; 69:1–5.
Article15. Bae S, Kim YG, Choi J, Hong J, Lee S, Kang T, Jeon H, Hong K, Kim E, Kwak A, Lee CK, Yoo B, Park YB, Song EY, Kim S. Elevated interleukin-32 expression in granulomatosis with polyangiitis. Rheumatology (Oxford). 2012; 51:1979–1988.
Article16. Jaekal J, Jhun H, Hong J, Park S, Lee J, Yoon D, Lee S, Her E, Yang Y, G Rho, Kim S. Cloning and characterization of bovine interleukin-32 beta isoform. Vet Immunol Immunopathol. 2010; 137:166–171.
Article17. Hong J, Bae S, Kang Y, Yoon D, Bai X, Chan ED, Azam T, Dinarello CA, Lee S, Her E, Rho G, Kim S. Suppressing IL-32 in monocytes impairs the induction of the proinflammatory cytokines TNFalpha and IL-1beta. Cytokine. 2010; 49:171–176.
Article18. Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005; 22:131–142.19. Marcondes AM, Li X, Tabellini L, Bartenstein M, Kabacka J, Sale GE, Hansen JA, Dinarello CA, Deeg HJ. Inhibition of IL-32 activation by alpha-1 antitrypsin suppresses alloreactivity and increases survival in an allogeneic murine marrow transplantation model. Blood. 2011; 118:5031–5039.
Article20. Lee S, Kim E, Jhun H, Hong J, Kwak A, Jo S, Bae S, Lee J, Kim B, Lee J, Youn S, Kim S, Kim M, Kim H, Lee Y, Choi DK, Kim YS, Kim S. Proinsulin shares a motif with interleukin-1alpha (IL-1alpha) and induces inflammatory cytokine via interleukin-1 receptor 1. J Biol Chem. 2016; 291:14620–14627.
Article21. Lee S, Lee Y, Hong K, Hong J, Bae S, Choi J, Jhun H, Kwak A, Kim E, Jo S, Dinarello CA. Effect of recombinant alpha1-antitrypsin Fc-fused (AAT-Fc) protein on the inhibition of inflammatory cytokine production and streptozotocin-induced diabetes. Mol Med. 2013; 19:65–71.
Article22. Choi J, Bae S, Hong J, Ryoo S, Jhun H, Hong K, Yoon D, Lee S, Her E, Choi W, Kim J, Azam T, Dinarello CA, Kim S. Paradoxical effects of constitutive human IL-32{gamma} in transgenic mice during experimental colitis. Proc Natl Acad Sci U S A. 2010; 107:21082–21086.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interleukin-32 in Inflammatory Autoimmune Diseases
- Anti-inflammatory effects of rutin in lipopolysaccharide-stimulated canine macrophage cells
- Effect of Nitric Oxide on the Viability of Bone Marrow - Derived Cultured Mast Cells
- Expression of Pro-inflammatory Cytokines during Distraction Osteogenesis of the Rat Tibia
- MiR-182-5p Mediated by Exosomes Derived From Bone Marrow Mesenchymal Stem Cell Attenuates Inflammatory Responses by Targeting TLR4 in a Mouse Model of Myocardial Infraction