Interleukin-32 in Inflammatory Autoimmune Diseases
- Affiliations
-
- 1Department of Biomedical Sciences and Technology, Konkuk University, Seoul 143-701, Korea. soohyun@konkuk.ac.kr
- KMID: 2168023
- DOI: http://doi.org/10.4110/in.2014.14.3.123
Abstract
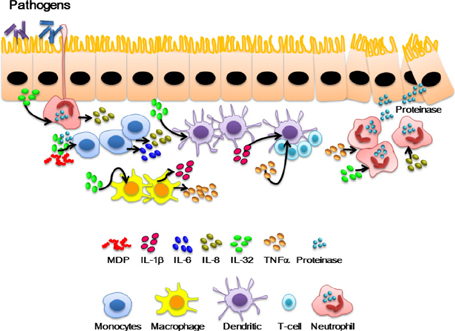
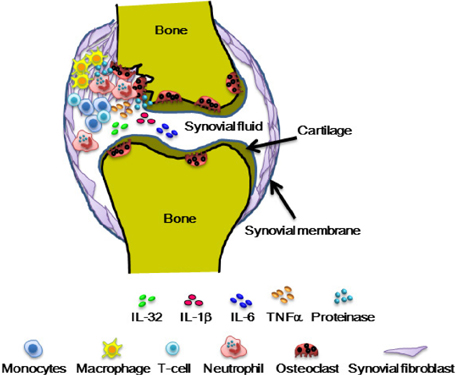
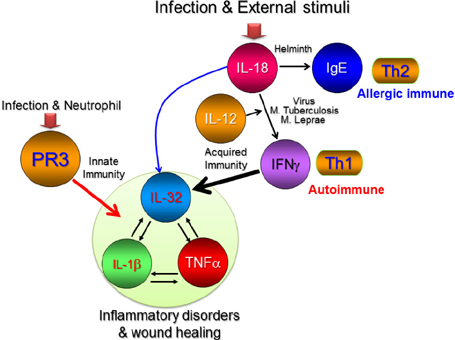
- Interleukin-32 (IL-32) is a cytokine inducing crucial inflammatory cytokines such as tumor necrosis factor-alpha (TNFalpha) and IL-6 and its expression is elevated in various inflammatory autoimmune diseases, certain cancers, as well as viral infections. IL-32 gene was first cloned from activated T cells, however IL-32 expression was also found in other immune cells and non-immune cells. IL-32 gene was identified in most mammals except rodents. It is transcribed as multiple-spliced variants in the absence of a specific activity of each isoform. IL-32 has been studied mostly in clinical fields such as infection, autoimmune, cancer, vascular disease, and pulmonary diseases. It is difficult to investigate the precise role of IL-32 in vivo due to the absence of IL-32 gene in mouse. The lack of mouse IL-32 gene restricts in vivo studies and restrains further development of IL-32 research in clinical applications although IL-32 new cytokine getting a spotlight as an immune regulatory molecule processing important roles in autoimmune, infection, and cancer. In this review, we discuss the regulation and function of IL-32 in inflammatory bowel diseases and rheumatoid arthritis.
Keyword
MeSH Terms
Figure
Cited by 5 articles
-
IL-32-induced Inflammatory Cytokines Are Selectively Suppressed by α1-antitrypsin in Mouse Bone Marrow Cells
Siyoung Lee, Dong-Ki Choi, Areum Kwak, Sinae Kim, Tam Thanh Nguyen, Gaae Gil, Eunhye Kim, Kwang Ha Yoo, In Ae Kim, Youngmin Lee, Hyunjhung Jhun, Edward D. Chan, Xiyuan Bai, Hyunwoo Kim, Yong-Sung Kim, Soohyun Kim
Immune Netw. 2017;17(2):116-120. doi: 10.4110/in.2017.17.2.116.Species Specific Antiviral Activity of Porcine Interferon-α8 (IFNα8)
Eunhye Kim, Hyunjhung Jhun, Joohee Kim, Unjoo Park, Seunghyun Jo, Areum Kwak, Sinae Kim, Tam T. Nguyen, Yongsun Kang, Insoo Choi, Joongbok Lee, Heijun Kim, Younghyun Kim, Siyoung Lee, Soohyun Kim
Immune Netw. 2017;17(6):424-436. doi: 10.4110/in.2017.17.6.424.Role of IL-32 Gamma on Bone Metabolism in Autoimmune Arthritis
Oh Chan Kwon, Soohyun Kim, Seokchan Hong, Chang-Keun Lee, Bin Yoo, Eun-Ju Chang, Yong-Gil Kim
Immune Netw. 2018;18(3):. doi: 10.4110/in.2018.18.e20.Structural Characteristics of Seven IL-32 Variants
Dong Hyun Sohn, Tam T. Nguyen, Sinae Kim, Saerok Shim, Siyoung Lee, Youngmin Lee, Hyunjhung Jhun, Tania Azam, Joohee Kim, Soohyun Kim
Immune Netw. 2019;19(2):. doi: 10.4110/in.2019.19.e8.Interleukin-32 Gamma as a New Face in Inflammatory Bone Diseases
Eun-Jin Lee, Bongkun Choi, Eui-Seung Hwang, Eun-Ju Chang
J Rheum Dis. 2017;24(1):14-20. doi: 10.4078/jrd.2017.24.1.14.
Reference
-
1. Netea MG, Azam T, Ferwerda G, Girardin SE, Walsh M, Park JS, Abraham E, Kim JM, Yoon DY, Dinarello CA, Kim SH. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2005; 102:16309–16314.
Article2. Imaeda H, Andoh A, Aomatsu T, Osaki R, Bamba S, Inatomi O, Shimizu T, Fujiyama Y. A new isoform of interleukin-32 suppresses IL-8 mRNA expression in the intestinal epithelial cell line HT-29. Mol Med Rep. 2011; 4:483–487.
Article3. Shioya M, Nishida A, Yagi Y, Ogawa A, Tsujikawa T, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y, Andoh A. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clin Exp Immunol. 2007; 149:480–486.
Article4. Ciccia F, Rizzo A, Accardo-Palumbo A, Giardina A, Bombardieri M, Guggino G, Taverna S, Leo GD, Alessandro R, Triolo G. Increased expression of interleukin-32 in the inflamed ileum of ankylosing spondylitis patients. Rheumatology (Oxford). 2012; 51:1966–1972.
Article5. Andoh A, Yagi Y, Shioya M, Nishida A, Tsujikawa T, Fujiyama Y. Mucosal cytokine network in inflammatory bowel disease. World J Gastroenterol. 2008; 14:5154–5161.
Article6. Fantini MC, Monteleone G, Macdonald TT. New players in the cytokine orchestra of inflammatory bowel disease. Inflamm Bowel Dis. 2007; 13:1419–1423.
Article7. Felaco P, Castellani ML, De Lutiis MA, Felaco M, Pandolfi F, Salini V, De Amicis D, Vecchiet J, Tete S, Ciampoli C, Conti F, Cerulli G, Caraffa A, Antinolfi P, Cuccurullo C, Perrella A, Theoharides TC, Conti P, Toniato E, Kempuraj D, Shaik YB. IL-32: a newly-discovered proinflammatory cytokine. J Biol Regul Homeost Agents. 2009; 23:141–147.8. Choi J, Bae S, Hong J, Ryoo S, Jhun H, Hong K, Yoon D, Lee S, Her E, Choi W, Kim J, Azam T, Dinarello CA, Kim S. Paradoxical effects of constitutive human IL-32{gamma} in transgenic mice during experimental colitis. Proc Natl Acad Sci U S A. 2010; 107:21082–21086.
Article9. Netea MG, Lewis EC, Azam T, Joosten LA, Jaekal J, Bae SY, Dinarello CA, Kim SH. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc Natl Acad Sci U S A. 2008; 105:3515–3520.
Article10. Choi JD, Bae SY, Hong JW, Azam T, Dinarello CA, Her E, Choi WS, Kim BK, Lee CK, Yoon DY, Kim SJ, Kim SH. Identification of the most active interleukin-32 isoform. Immunology. 2009; 126:535–542.
Article11. Kim YG, Lee CK, Oh JS, Kim SH, Kim KA, Yoo B. Effect of interleukin-32gamma on differentiation of osteoclasts from CD14+ monocytes. Arthritis Rheum. 2010; 62:515–523.12. Moon YM, Yoon BY, Her YM, Oh HJ, Lee JS, Kim KW, Lee SY, Woo YJ, Park KS, Park SH, Kim HY, Cho ML. IL-32 and IL-17 interact and have the potential to aggravate osteoclastogenesis in rheumatoid arthritis. Arthritis Res Ther. 2012; 14:R246.
Article13. Xu WD, Zhang M, Feng CC, Yang XK, Pan HF, Ye DQ. IL-32 with potential insights into rheumatoid arthritis. Clin Immunol. 2013; 147:89–94.
Article14. Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR, Barrera P, van de Loo FA, Dinarello CA, van den Berg WB. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2006; 103:3298–3303.
Article15. Gui M, Zhang H, Zhong K, Li Y, Sun J, Wang L. Clinical significance of interleukin-32 expression in patients with rheumatoid arthritis. Asian Pac J Allergy Immunol. 2013; 31:73–78.16. Jeong HJ, Oh HA, Lee BJ, Kim HM. Inhibition of IL-32 and TSLP production through the attenuation of caspase-1 activation in an animal model of allergic rhinitis by Naju Jjok (Polygonum tinctorium). Int J Mol Med. 2014; 33:142–150.
Article17. Cagnard N, Letourneur F, Essabbani A, Devauchelle V, Mistou S, Rapinat A, Decraene C, Fournier C, Chiocchia G. Interleukin-32, CCL2, PF4F1 and GFD10 are the only cytokine/chemokine genes differentially expressed by in vitro cultured rheumatoid and osteoarthritis fibroblast-like synoviocytes. Eur Cytokine Netw. 2005; 16:289–292.18. Zivojinovic SM, Pejnovic NN, Sefik-Bukilica MN, Kovacevic LV, Soldatovic II, Damjanov NS. Tumor necrosis factor blockade differentially affects innate inflammatory and Th17 cytokines in rheumatoid arthritis. J Rheumatol. 2012; 39:18–21.
Article19. Heinhuis B, Koenders MI, van Riel PL, van de Loo FA, Dinarello CA, Netea MG, van den Berg WB, Joosten LA. Tumour necrosis factor alpha-driven IL-32 expression in rheumatoid arthritis synovial tissue amplifies an inflammatory cascade. Ann Rheum Dis. 2011; 70:660–667.
Article20. Heinhuis B, Koenders MI, van de Loo FA, Netea MG, van den Berg WB, Joosten LA. Inflammation-dependent secretion and splicing of IL-32{gamma} in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2011; 108:4962–4967.
Article21. Alsaleh G, Sparsa L, Chatelus E, Ehlinger M, Gottenberg JE, Wachsmann D, Sibilia J. Innate immunity triggers IL-32 expression by fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther. 2010; 12:R135.
Article22. Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005; 22:131–142.