Yonsei Med J.
2016 Sep;57(5):1252-1259. 10.3349/ymj.2016.57.5.1252.
Oxidative Stress-Activated NHE1 Is Involved in High Glucose-Induced Apoptosis in Renal Tubular Epithelial Cells
- Affiliations
-
- 1Department of Nephrology, Tianjin Union Medicine Center, Tianjin, China.
- 2Department of Rehabilitation, Tangdu Hospital, Fourth Military Medical University, Xi'an, China.
- 3Department of Cardiology, Tianjin Union Medicine Center, Tianjin, China. chunjiezhaocjz@163.com
- KMID: 2374173
- DOI: http://doi.org/10.3349/ymj.2016.57.5.1252
Abstract
- PURPOSE
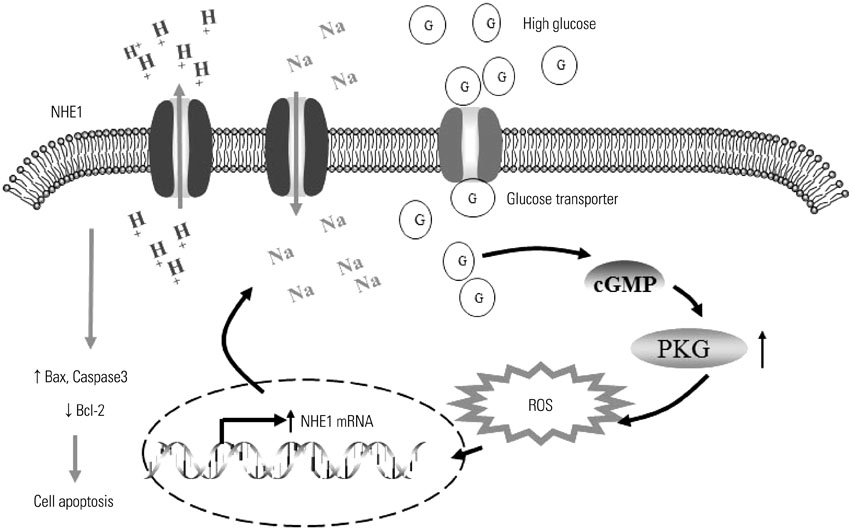
Diabetic nephropathy (DN) is a prevalent chronic microvascular complication of diabetes mellitus involving disturbances in electrolytes and the acid-base balance caused by a disorder of glucose metabolism. NHE1 is a Na+/H+ exchanger responsible for keeping intracellular pH (pHi) balance and cell growth. Our study aimed to investigate roles of NHE1 in high glucose (HG)-induced apoptosis in renal tubular epithelial cells.
MATERIALS AND METHODS
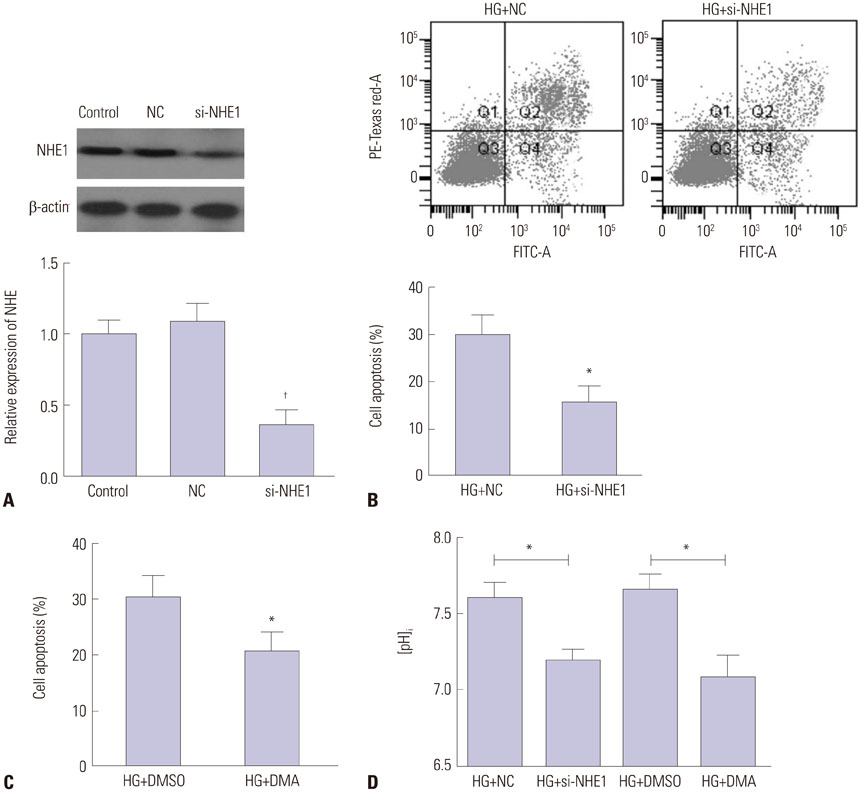
Renal epithelial tubular cell line HK-2 was cultured in medium containing 5 mM or 30 mM glucose. Then, cell apoptosis, oxidative stress, NHE1 expression, and pHi were evaluated. NHE1 siRNA and inhibitor were used to evaluate its role in cell apoptosis.
RESULTS
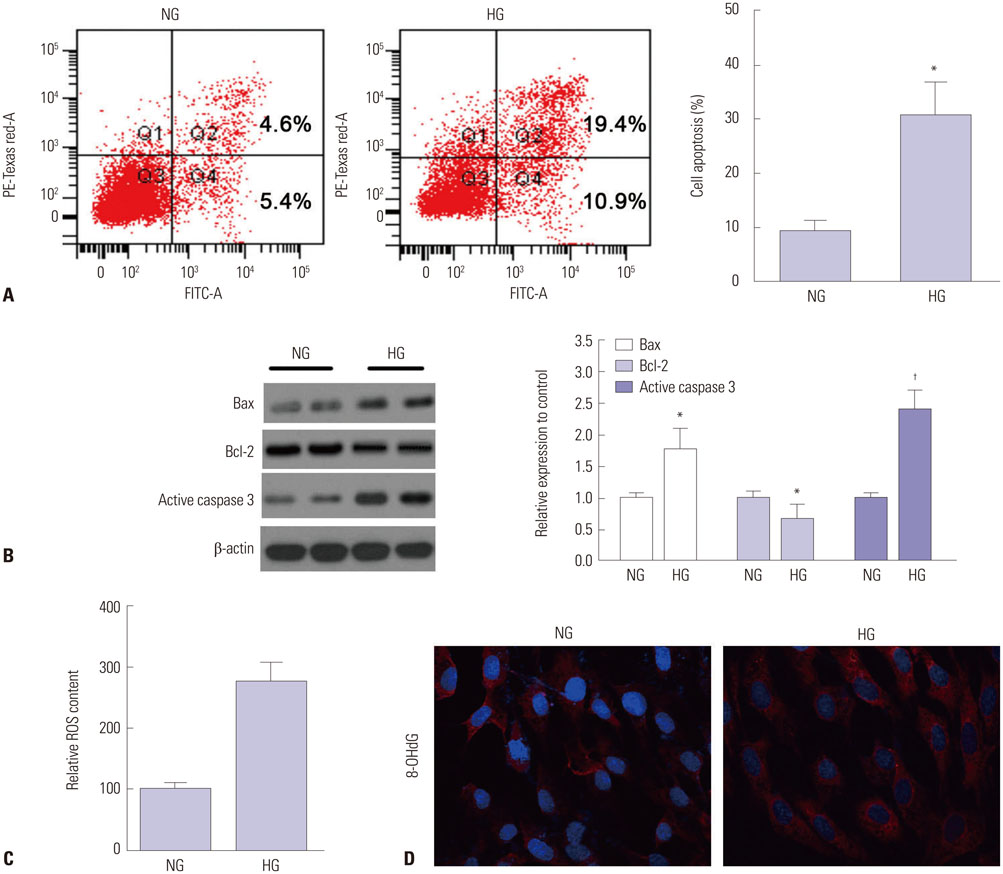
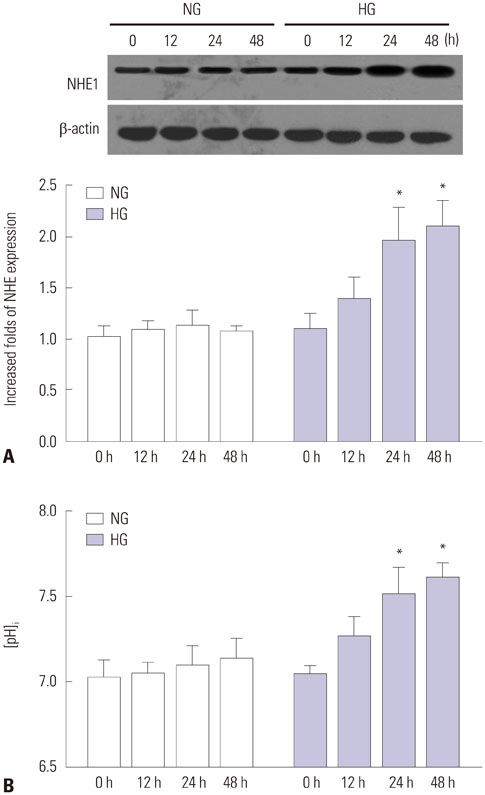
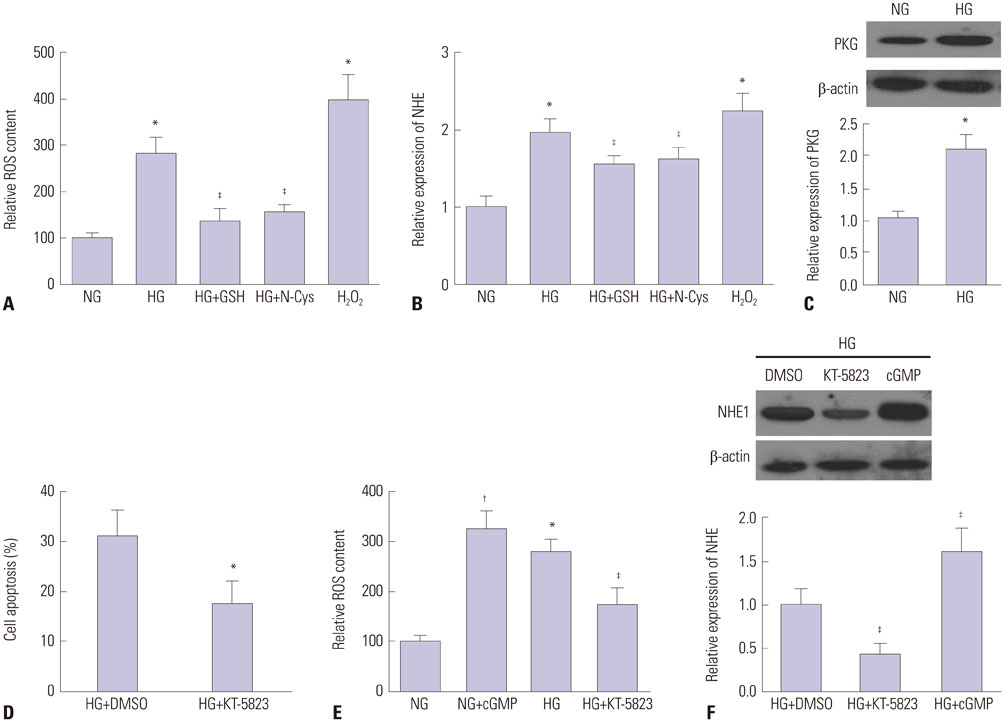
HG significantly increased cell apoptosis and the production of reactive oxygen species (ROS) and 8-OHdG (p<0.05). Meanwhile, we found that HG induced the expression of NHE1 and increased the pHi from 7.0 to 7.6 after 48 h of incubation. However, inhibiting NHE1 using its specific siRNA or antagonist DMA markedly reduced cell apoptosis stimulated by HG. In addition, suppressing cellular oxidative stress using antioxidants, such as glutathione and N-acetyl cysteine, significantly reduced the production of ROS, accompanied by a decrease in NHE1. We also found that activated cyclic GMP-Dependent Protein Kinase Type I (PKG) signaling promoted the production of ROS, which contributed to the regulation of NHE1 functions.
CONCLUSION
Our study indicated that HG activates PKG signaling and elevates the production of ROS, which was responsible for the induction of NHE1 expression and dysfunction, as well as subsequent cell apoptosis, in renal tubular epithelial cells.
Keyword
MeSH Terms
-
Antioxidants/metabolism
Apoptosis/*drug effects
Cation Transport Proteins/*metabolism
Cell Cycle/drug effects
Cell Line
Dose-Response Relationship, Drug
Epithelial Cells/*cytology/drug effects/*metabolism
Glucose/*pharmacology
Glutathione/metabolism
Humans
Kidney Tubules/*cytology
Oxidative Stress/*drug effects
Reactive Oxygen Species/metabolism
Signal Transduction/drug effects
Sodium-Hydrogen Antiporter/*metabolism
Antioxidants
Cation Transport Proteins
Glucose
Glutathione
Reactive Oxygen Species
Sodium-Hydrogen Antiporter
Figure
Reference
-
1. Okada H, Senmaru T, Fukui M, Kondo Y, Ishigami A, Maruyama N, et al. Senescence marker protein-30/gluconolactonase deficiency exacerbates diabetic nephropathy through tubular injury in a mouse model of type 1 diabetes. J Diabetes Investig. 2015; 6:35–43.
Article2. Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem. 2004; 259:67–70.
Article3. Karki P, Fliegel L. Overexpression of the NHE1 isoform of the Na(+)/H (+) exchanger causes elevated apoptosis in isolated cardiomyocytes after hypoxia/reoxygenation challenge. Mol Cell Biochem. 2010; 338:47–57.
Article4. Ng LL, Davies JE, Siczkowski M, Sweeney FP, Quinn PA, Krolewski B, et al. Abnormal Na+/H+ antiporter phenotype and turnover of immortalized lymphoblasts from type 1 diabetic patients with nephropathy. J Clin Invest. 1994; 93:2750–2757.
Article5. Li P, Chen GR, Wang F, Xu P, Liu LY, Yin YL, et al. Inhibition of NA(+)/H(+) exchanger 1 attenuates renal dysfunction induced by advanced glycation end products in rats. J Diabetes Res. 2016; 2016:1802036.6. Schelling JR, Abu Jawdeh BG. Regulation of cell survival by Na+/H+ exchanger-1. Am J Physiol Renal Physiol. 2008; 295:F625–F632.7. Karydis A, Jimenez-Vidal M, Denker SP, Barber DL. Mislocalized scaffolding by the Na-H exchanger NHE1 dominantly inhibits fibronectin production and TGF-beta activation. Mol Biol Cell. 2009; 20:2327–2336.
Article8. Vallés PG, Bocanegra V, Gil Lorenzo A, Costantino VV. Physiological functions and regulation of the Na+/H+ exchanger [NHE1] in renal tubule epithelial cells. Kidney Blood Press Res. 2015; 40:452–466.
Article9. Sotirakopoulos N, Kalogiannidou I, Tersi M, Armentzioiou K, Sivridis D, Mavromatidis K. Acid-base and electrolyte disorders in patients with diabetes mellitus. Saudi J Kidney Dis Transpl. 2012; 23:58–62.10. Marchelek-Myśliwiec M, Cichocka E, Dziedziejko V, Dutkiewicz GŻ, Stȩpniewska J, Safranow K, et al. Insulin resistance and brainderived neurotrophic factor levels in chronic kidney disease. Ann Clin Biochem. 2015; 52(Pt 2):213–219.
Article11. Focaccetti C, Bruno A, Magnani E, Bartolini D, Principi E, Dallaglio K, et al. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS One. 2015; 10:e0115686.
Article12. Huang C, Wang J, Chen Z, Wang Y, Zhang W. 2-phenylethynesulfonamide prevents induction of pro-inflammatory factors and attenuates LPS-induced liver injury by targeting NHE1-Hsp70 complex in mice. PLoS One. 2013; 8:e67582.
Article13. Hong NJ, Garvin JL. Endogenous flow-induced nitric oxide reduces superoxide-stimulated Na/H exchange activity via PKG in thick ascending limbs. Am J Physiol Renal Physiol. 2015; 308:F444–F449.
Article14. Huang YP, Gao FF, Wang B, Zheng FC, Zhang YM, Chen YC, et al. N-n-butyl haloperidol iodide inhibits H2O2-induced Na+/Ca2+-exchanger activation via the Na+/H+ exchanger in rat ventricular myocytes. Drug Des Devel Ther. 2014; 8:1257–1267.15. van den Berg E, Hospers FA, Navis G, Engberink MF, Brink EJ, Geleijnse JM, et al. Dietary acid load and rapid progression to endstage renal disease of diabetic nephropathy in Westernized South Asian people. J Nephrol. 2011; 24:11–17.
Article16. Pannabecker TL, Brokl OH, Kim YK, Abbott DE, Dantzler WH. Regulation of intracellular pH in rat renal inner medullary thin limbs of Henle's loop. Pflugers Arch. 2002; 443:446–457.
Article17. Konstantinidis D, Paletas K, Koliakos G, Kaloyianni M. The ambiguous role of the Na+-H+ exchanger isoform 1 (NHE1) in leptin-induced oxidative stress in human monocytes. Cell Stress Chaperones. 2009; 14:591–601.
Article18. Abu Jawdeh BG, Khan S, Deschênes I, Hoshi M, Goel M, Lock JT, et al. Phosphoinositide binding differentially regulates NHE1 Na+/H+ exchanger-dependent proximal tubule cell survival. J Biol Chem. 2011; 286:42435–42445.
Article19. Ebrahimpour Koujan S, Gargari BP, Mobasseri M, Valizadeh H, Asghari-Jafarabadi M. Effects of Silybum marianum (L.) Gaertn. (silymarin) extract supplementation on antioxidant status and hs-CRP in patients with type 2 diabetes mellitus: a randomized, triple-blind, placebo-controlled clinical trial. Phytomedicine. 2015; 22:290–296.
Article20. Hou S, Zheng F, Li Y, Gao L, Zhang J. The protective effect of glycyrrhizic acid on renal tubular epithelial cell injury induced by high glucose. Int J Mol Sci. 2014; 15:15026–15043.
Article21. Dang J, Jia R, Tu Y, Xiao S, Ding G. Erythropoietin prevents reactive oxygen species generation and renal tubular cell apoptosis at high glucose level. Biomed Pharmacother. 2010; 64:681–685.
Article22. He T, Guan X, Wang S, Xiao T, Yang K, Xu X, et al. Resveratrol prevents high glucose-induced epithelial-mesenchymal transition in renal tubular epithelial cells by inhibiting NADPH oxidase/ROS/ERK pathway. Mol Cell Endocrinol. 2015; 402:13–20.
Article23. Ferreira R, Wong R, Schlichter LC. KCa3.1/IK1 Channel Regulation by cGMP-Dependent Protein Kinase (PKG) via Reactive Oxygen Species and CaMKII in Microglia: An Immune Modulating Feedback System? Front Immunol. 2015; 6:153.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Oligomeric proanthocyanidin ameliorates sepsis-associated renal tubular injury: involvement of oxidative stress, inflammation, PI3K/AKT and NFκκB signaling pathways
- Regulation of Angiotensin II Binding in Renal Proximal Tubule Cells by High Glucose : I.Involvement of PKC, Ca2+, and Oxidative Stress
- Sweroside plays a role in mitigating high glucose-induced damage in human renal tubular epithelial HK-2 cells by regulating the SIRT1/NF-κB signaling pathway
- Characterization of Cigarette Smoke Extract (CSE)-induced Cell Death in Lung Epithelial Cells
- Padina arborescens extract protects high glucose-induced apoptosis in pancreatic beta cells by reducing oxidative stress