Nutr Res Pract.
2014 Oct;8(5):494-500. 10.4162/nrp.2014.8.5.494.
Padina arborescens extract protects high glucose-induced apoptosis in pancreatic beta cells by reducing oxidative stress
- Affiliations
-
- 1Department of Food and Nutrition, College of Medical and Life Science, Silla University, Busan 617-736, Korea.
- 2Department of Food Science and Nutrition, Pusan National University, 2, Busandaehak-ro 63beon-gil, Geumjeong-gu, Busan 609-735, Korea. hanjs@pusan.ac.kr
- KMID: 2313769
- DOI: http://doi.org/10.4162/nrp.2014.8.5.494
Abstract
- BACKGROUND/OBJECTIVES
This study investigated whether Padina arborescens extract (PAE) protects INS-1 pancreatic beta cells against glucotoxicity-induced apoptosis.
MATERIALS/METHODS
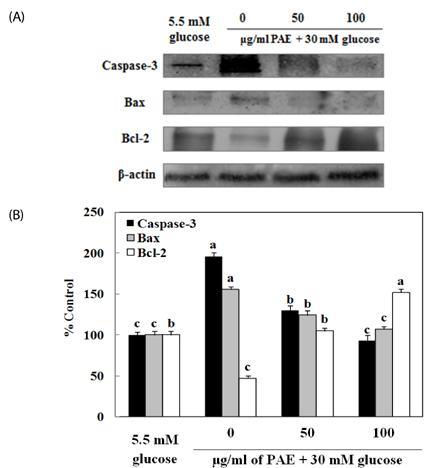
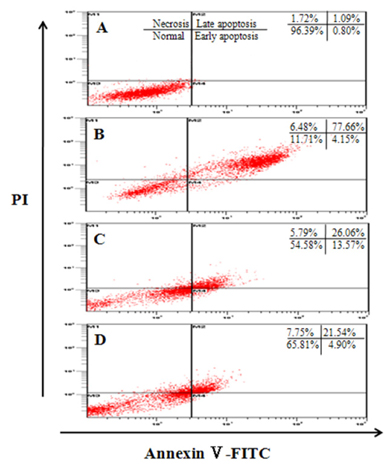
Assays, including cell viability, lipid peroxidation, generation of intracellular ROS, NO production, antioxidant enzyme activity and insulin secretion, were conducted. The expressions of Bax, Bcl-2, and caspase-3 proteins in INS-1 cells were evaluated by western blot analysis, and apoptosis/necrosis induced by high glucose was determined by analysis of FITC-Annexin V/PI staining.
RESULTS
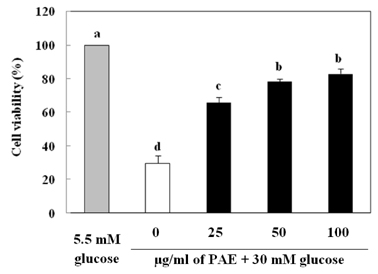
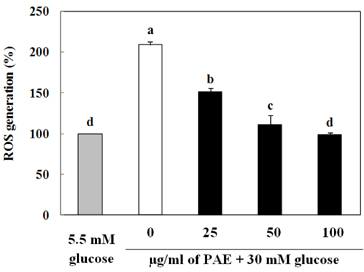
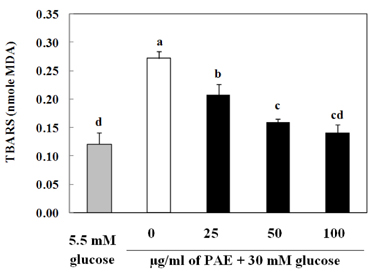
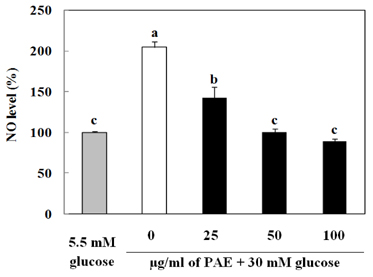
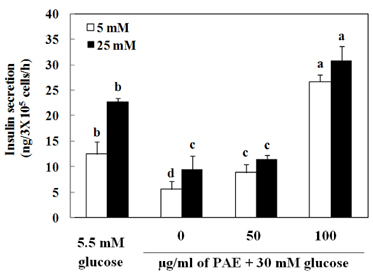
Treatment with high concentrations of glucose induced INS-1 cell death, but PAE at concentrations of 25, 50 or 100 microg/ml significantly increased cell viability. The treatment with PAE dose dependently reduced the lipid peroxidation and increased the activities of antioxidant enzymes reduced by 30 mM glucose, while intracellular ROS levels increased under conditions of 30 mM glucose. PAE treatment improved the secretory responsiveness following stimulation with glucose. The results also demonstrated that glucotoxicity-induced apoptosis is associated with modulation of the Bax/Bcl-2 ratio. When INS-1 cells were stained with Annexin V/PI, we found that PAE reduced apoptosis by glucotoxicity.
CONCLUSIONS
In conclusion, the present study indicates that PAE protects against high glucose-induced apoptosis in pancreatic beta cells by reducing oxidative stress.
Keyword
MeSH Terms
Figure
Reference
-
1. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004; 279:42351–42354.
Article2. Zúrová-Nedelcevová J, Navarová J, Drábiková K, Jancinová V, Petríková M, Bernátová I, Kristová V, Snirc V, Nosál'ová V, Sotníková R. Participation of reactive oxygen species in diabetes-induced endothelial dysfunction. Neuro Endocrinol Lett. 2006; 27:Suppl 2. 168–171.3. Mandrup-Poulsen T, Helqvist S, Wogensen LD, Mølvig J, Pociot F, Johannesen J, Nerup J. Cytokine and free radicals as effector molecules in the destruction of pancreatic beta cells. Curr Top Microbiol Immunol. 1990; 164:169–193.4. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003; 52:102–110.
Article5. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005; 54:1615–1625.6. Heo SJ, Park EJ, Lee KW, Jeon YJ. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour Technol. 2005; 96:1613–1623.
Article7. Pal Singh I, Bharate SB. Phloroglucinol compounds of natural origin. Nat Prod Rep. 2006; 23:558–591.
Article8. Park MH, Han JS. Protective effect of Padina arborescens extract against high glucose-induced oxidative damage in human umbilical vein endothelial cells. Prev Nutr Food Sci. 2013; 18:11–17.
Article9. Park MH, Han JS. Hypoglycemic effect of Padina arborescens extract in streptozotocin-induced diabetic mice. Prev Nutr Food Sci. 2012; 17:239–244.
Article10. Fautz R, Husein B, Hechenberger C. Application of the neutral red assay (NR assay) to monolayer cultures of primary hepatocytes: rapid colorimetric viability determination for the unscheduled DNA synthesis test (UDS). Mutat Res. 1991; 253:173–179.
Article11. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999; 27:612–616.
Article12. Fraga CG, Leibovitz BE, Tappel AL. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med. 1988; 4:155–161.
Article13. Nath J, Powledge A. Modulation of human neutrophil inflammatory responses by nitric oxide: studies in unprimed and LPS-primed cells. J Leukoc Biol. 1997; 62:805–816.
Article14. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974; 47:469–474.
Article15. Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105:121–126.16. Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976; 71:952–958.
Article17. Green CD, Jump DB, Olson LK. Elevated insulin secretion from liver X receptor-activated pancreatic β-cells involves increased de novo lipid synthesis and triacylglyceride turnover. Endocrinology. 2009; 150:2637–2645.
Article18. Yamabe N, Kang KS, Goto E, Tanaka T, Yokozawa T. Beneficial effect of Corni Fructus, a constituent of Hachimi-jio-gan, on advanced glycation end-product-mediated renal injury in streptozotocin-treated diabetic rats. Biol Pharm Bull. 2007; 30:520–526.
Article19. Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans. 2008; 36:343–347.20. Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004; 53:Suppl 1. S119–S124.21. Tiedge M, Lortz S, Munday R, Lenzen S. Complementary action of antioxidant enzymes in the protection of bioengineered insulin-producing RINm5F cells against the toxicity of reactive oxygen species. Diabetes. 1998; 47:1578–1585.
Article22. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002; 23:599–622.
Article23. Del Guerra S, Grupillo M, Masini M, Lupi R, Bugliani M, Torri S, Boggi U, Del Chiaro M, Vistoli F, Mosca F, Del Prato S, Marchetti P. Gliclazide protects human islet beta-cells from apoptosis induced by intermittent high glucose. Diabetes Metab Res Rev. 2007; 23:234–238.
Article24. Robertson R, Zhou H, Zhang T, Harmon JS. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem Biophys. 2007; 48:139–146.
Article25. Kong Q, Lin CL. Oxidative damage to RNA: mechanisms, consequences, and diseases. Cell Mol Life Sci. 2010; 67:1817–1829.
Article26. Kappus H. Oxidative stress in chemical toxicity. Arch Toxicol. 1987; 60:144–149.
Article27. Du X, Stocklauser-Färber K, Rösen P. Generation of reactive oxygen intermediates, activation of NF-kappaB, and induction of apoptosis in human endothelial cells by glucose: role of nitric oxide synthase? Free Radic Biol Med. 1999; 27:752–763.
Article28. Messmer UK, Reed UK, Brüne B. Bcl-2 protects macrophages from nitric oxide-induced apoptosis. J Biol Chem. 1996; 271:20192–20197.
Article29. McDaniel ML, Kwon G, Hill JR, Marshall CA, Corbett JA. Cytokines and nitric oxide in islet inflammation and diabetes. Proc Soc Exp Biol Med. 1996; 211:24–32.
Article30. Kaneto H, Fujii J, Myint T, Miyazawa N, Islam KN, Kawasaki Y, Suzuki K, Nakamura M, Tatsumi H, Yamasaki Y, Taniguchi N. Reducing sugars trigger oxidative modification and apoptosis in pancreatic beta-cells by provoking oxidative stress through the glycation reaction. Biochem J. 1996; 320:855–863.
Article31. Tajiri Y, Grill V. Aminoguanidine exerts a beta-cell function-preserving effect in high glucose-cultured beta-cells (INS-1). Int J Exp Diabetes Res. 2000; 1:111–119.
Article32. Grankvist K, Marklund S, Täljedal IB. Superoxide dismutase is a prophylactic against alloxan diabetes. Nature. 1981; 294:158–160.
Article33. Benhamou PY, Moriscot C, Richard MJ, Beatrix O, Badet L, Pattou F, Kerr-Conte J, Chroboczek J, Lemarchand P, Halimi S. Adenovirus-mediated catalase gene transfer reduces oxidant stress in human, porcine and rat pancreatic islets. Diabetologia. 1998; 41:1093–1100.
Article34. Krause MS, McClenaghan NH, Flatt PR, de Bittencourt PI, Murphy C, Newsholme P. L-arginine is essential for pancreatic beta-cell functional integrity, metabolism and defense from inflammatory challenge. J Endocrinol. 2011; 211:87–97.
Article35. Moriscot C, Pattou F, Kerr-Conte J, Richard MJ, Lemarchand P, Benhamou PY. Contribution of adenoviral-mediated superoxide dismutase gene transfer to the reduction in nitric oxide-induced cytotoxicity on human islets and INS-1 insulin-secreting cells. Diabetologia. 2000; 43:625–631.
Article36. Fariss MW, Chan CB, Patel M, Van Houten B, Orrenius S. Role of mitochondria in toxic oxidative stress. Mol Interv. 2005; 5:94–111.
Article37. Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000; 256:50–57.
Article38. Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999; 6:99–104.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High glucose stimulates glutamate uptakes in pancreatic beta-cells
- Oxidative Stress in Pancreatic Islet beta-cells Exposed to High Glucose Concentration
- Testosterone Protects Pancreatic β-cells from Apoptosis and Stress-Induced Accelerated Senescence
- Decreased Expression and Induced Nucleocytoplasmic Translocation of Pancreatic and Duodenal Homeobox 1 in INS-1 Cells Exposed to High Glucose and Palmitate
- Paeoniflorin Protects Retinal Pigment Epithelial Cells from High Glucose-Induced Oxidative Damage by Activating Nrf2-Mediated HO-1 Signaling