Diabetes Metab J.
2011 Feb;35(1):65-71. 10.4093/dmj.2011.35.1.65.
Decreased Expression and Induced Nucleocytoplasmic Translocation of Pancreatic and Duodenal Homeobox 1 in INS-1 Cells Exposed to High Glucose and Palmitate
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. kihos@catholic.ac.kr
- KMID: 2281494
- DOI: http://doi.org/10.4093/dmj.2011.35.1.65
Abstract
- BACKGROUND
Type 2 diabetes mellitus (T2DM) is often accompanied by increased levels of circulating fatty acid. Elevations in fatty acids and glucose for prolonged periods of time have been suggested to cause progressive dysfunction or apoptosis of pancreatic beta cells in T2DM. However, the precise mechanism of this adverse effect is not well understood.
METHODS
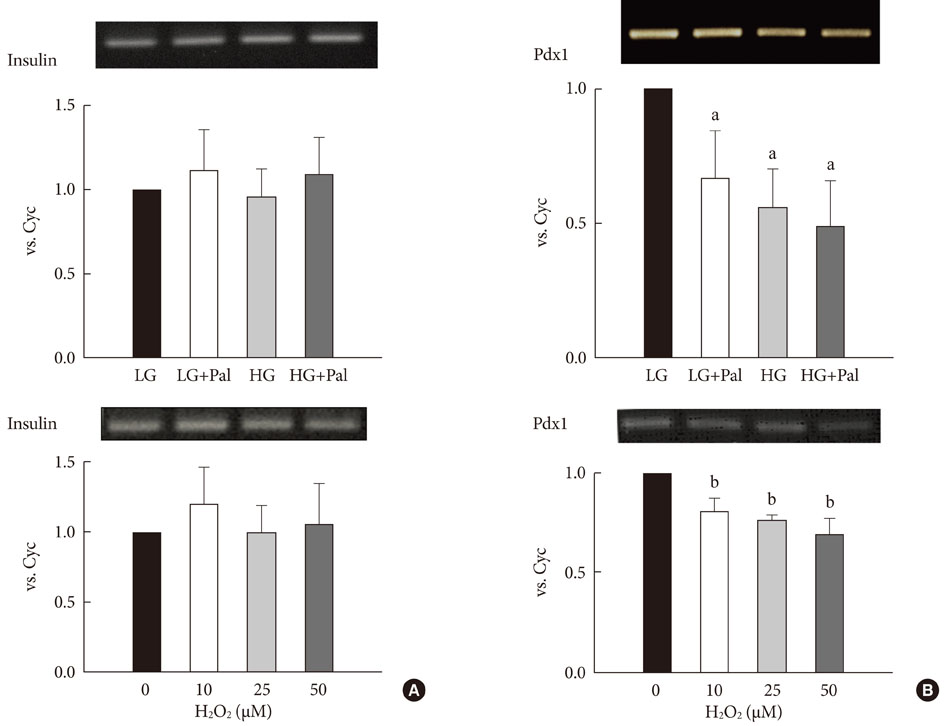
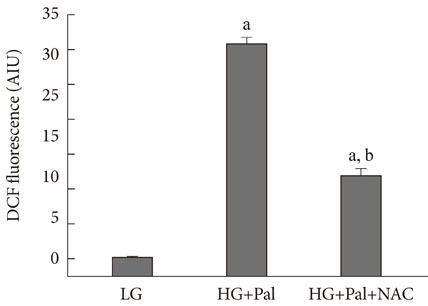
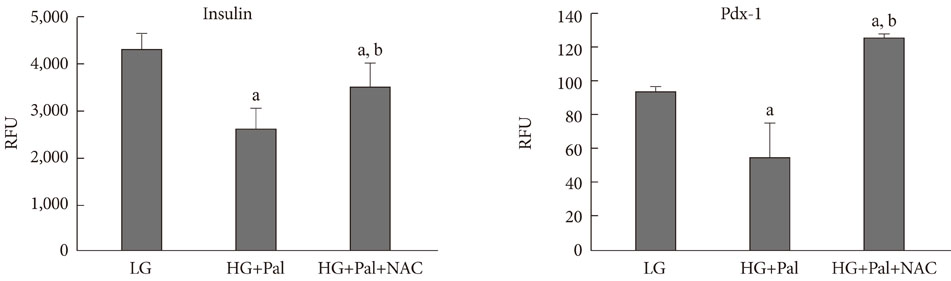
INS-1 rat-derived insulin-secreting cells were exposed to 30 mM glucose and 0.25 mM palmitate for 48 hours.
RESULTS
The production of reactive oxygen species increased significantly. Pancreatic and duodenal homeobox 1 (Pdx1) expression was down-regulated, as assessed by reverse transcription-polymerase chain reaction and Western blot analyses. The promoter activities of insulin and Pdx1 were also diminished. Of note, there was nucleocytoplasmic translocation of Pdx1, which was partially prevented by treatment with an antioxidant, N-acetyl-L-cysteine.
CONCLUSION
Our data suggest that prolonged exposure of beta cells to elevated levels of glucose and palmitate negatively affects Pdx1 expression via oxidative stress.
Keyword
MeSH Terms
Figure
Reference
-
1. Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006. 116:1802–1812.2. Robertson RP. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr Opin Pharmacol. 2006. 6:615–619.3. Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003. 52:581–587.4. Poitout V, Robertson RP. Minireview: secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002. 143:339–342.5. Hay CW, Docherty K. Comparative analysis of insulin gene promoters: implications for diabetes research. Diabetes. 2006. 55:3201–3213.6. Mounier C, Posner BI. Transcriptional regulation by insulin: from the receptor to the gene. Can J Physiol Pharmacol. 2006. 84:713–724.7. German M, Ashcroft S, Docherty K, Edlund H, Edlund T, Goodison S, Imura H, Kennedy G, Madsen O, Melloul D. The insulin gene promoter. A simplified nomenclature. Diabetes. 1995. 44:1002–1004.8. German MS, Wang J, Chadwick RB, Rutter WJ. Synergistic activation of the insulin gene by a LIM-homeo domain protein and a basic helix-loop-helix protein: building a functional insulin minienhancer complex. Genes Dev. 1992. 6:2165–2176.9. Glick E, Leshkowitz D, Walker MD. Transcription factor BETA2 acts cooperatively with E2A and PDX1 to activate the insulin gene promoter. J Biol Chem. 2000. 275:2199–2204.10. Qiu Y, Guo M, Huang S, Stein R. Insulin gene transcription is mediated by interactions between the p300 coactivator and PDX-1, BETA2, and E47. Mol Cell Biol. 2002. 22:412–420.11. Ohneda K, Mirmira RG, Wang J, Johnson JD, German MS. The homeodomain of PDX-1 mediates multiple protein-protein interactions in the formation of a transcriptional activation complex on the insulin promoter. Mol Cell Biol. 2000. 20:900–911.12. Hagman DK, Hays LB, Parazzoli SD, Poitout V. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem. 2005. 280:32413–32418.13. Wang H, Iezzi M, Theander S, Antinozzi PA, Gauthier BR, Halban PA, Wollheim CB. Suppression of Pdx-1 perturbs proinsulin processing, insulin secretion and GLP-1 signalling in INS-1 cells. Diabetologia. 2005. 48:720–731.14. Kawamori D, Kaneto H, Nakatani Y, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem. 2006. 281:1091–1098.15. Rafiq I, Kennedy HJ, Rutter GA. Glucose-dependent translocation of insulin promoter factor-1 (IPF-1) between the nuclear periphery and the nucleoplasm of single MIN6 beta-cells. J Biol Chem. 1998. 273:23241–23247.16. Poitout V, Hagman D, Stein R, Artner I, Robertson RP, Harmon JS. Regulation of the insulin gene by glucose and fatty acids. J Nutr. 2006. 136:873–876.17. Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983. 11:1475–1489.18. Ko SH, Ryu GR, Kim S, Ahn YB, Yoon KH, Kaneto H, Ha H, Kim YS, Song KH. Inducible nitric oxide synthase-nitric oxide plays an important role in acute and severe hypoxic injury to pancreatic beta cells. Transplantation. 2008. 85:323–330.19. Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996. 20:463–466.20. Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997. 46:1733–1742.21. Kaneto H, Matsuoka TA, Nakatani Y, Kawamori D, Matsuhisa M, Yamasaki Y. Oxidative stress and the JNK pathway in diabetes. Curr Diabetes Rev. 2005. 1:65–72.22. Kawamori D, Kajimoto Y, Kaneto H, Umayahara Y, Fujitani Y, Miyatsuka T, Watada H, Leibiger IB, Yamasaki Y, Hori M. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes. 2003. 52:2896–2904.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Involvement of Estrogen Receptor-α in the Activation of Nrf2-Antioxidative Signaling Pathways by Silibinin in Pancreatic β-Cells
- The Glucotoxicity Protecting Effect of Ezetimibe in Pancreatic Beta Cells via Inhibition of CD36
- A Protective Role for Heme Oxygenase-1 in INS-1 Cells and Rat Islets that are Exposed to High Glucose Conditions
- Hexane Extract of Orthosiphon stamineus Induces Insulin Expression and Prevents Glucotoxicity in INS-1 Cells
- Protective Effect of Heme Oxygenase-1 on High Glucose-Induced Pancreatic beta-Cell Injury