Korean J Physiol Pharmacol.
2023 Nov;27(6):533-540. 10.4196/kjpp.2023.27.6.533.
Sweroside plays a role in mitigating high glucose-induced damage in human renal tubular epithelial HK-2 cells by regulating the SIRT1/NF-κB signaling pathway
- Affiliations
-
- 1Department of Endocrinology, Second Hospital, Shanxi Medical University, Taiyuan, Shanxi 030001, China
- 2Department of Endocrinology, Changzhi People’s Hospital Affiliated to Changzhi Medical College, Changzhi, Shanxi 046000, China
- KMID: 2547319
- DOI: http://doi.org/10.4196/kjpp.2023.27.6.533
Abstract
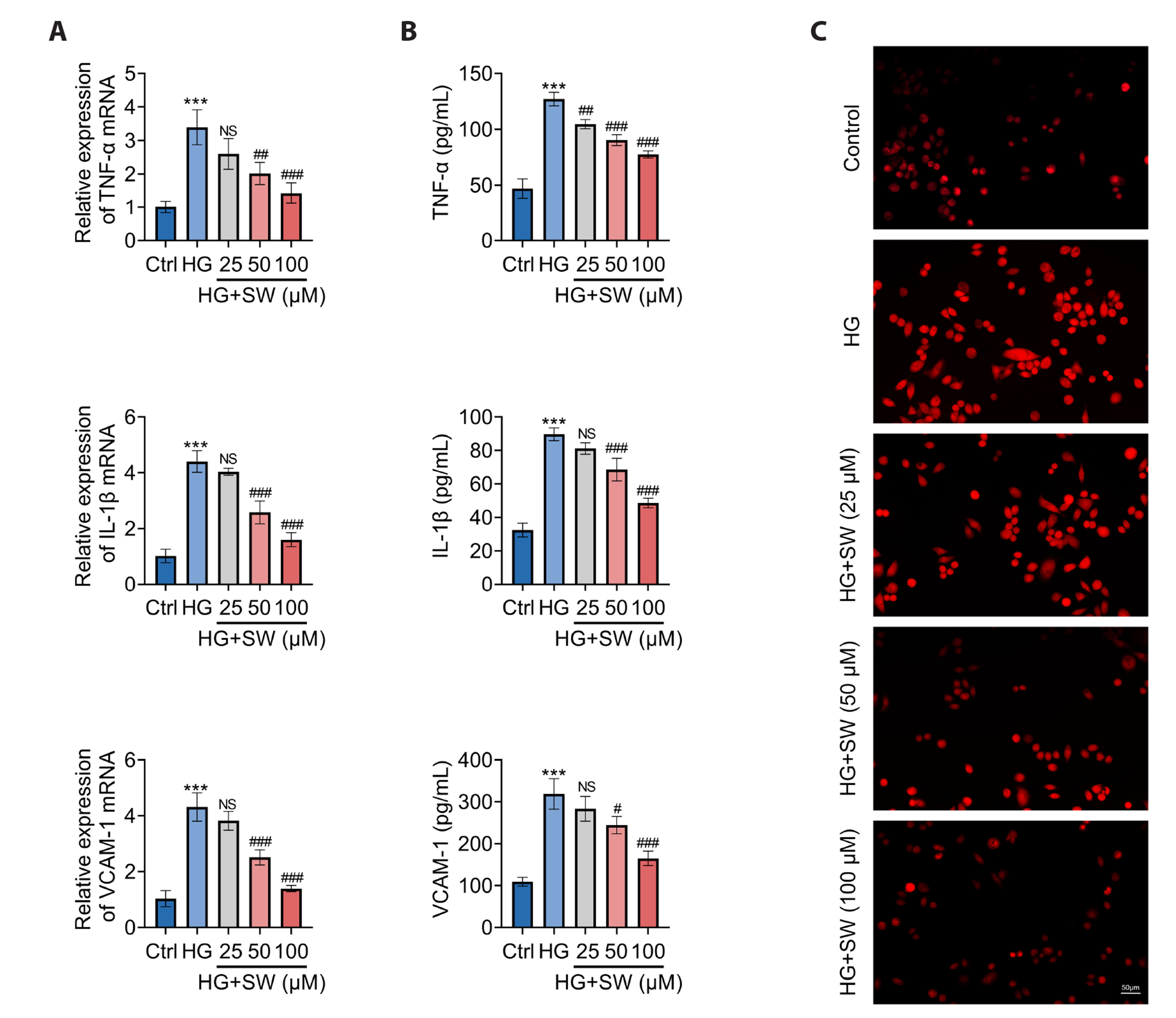
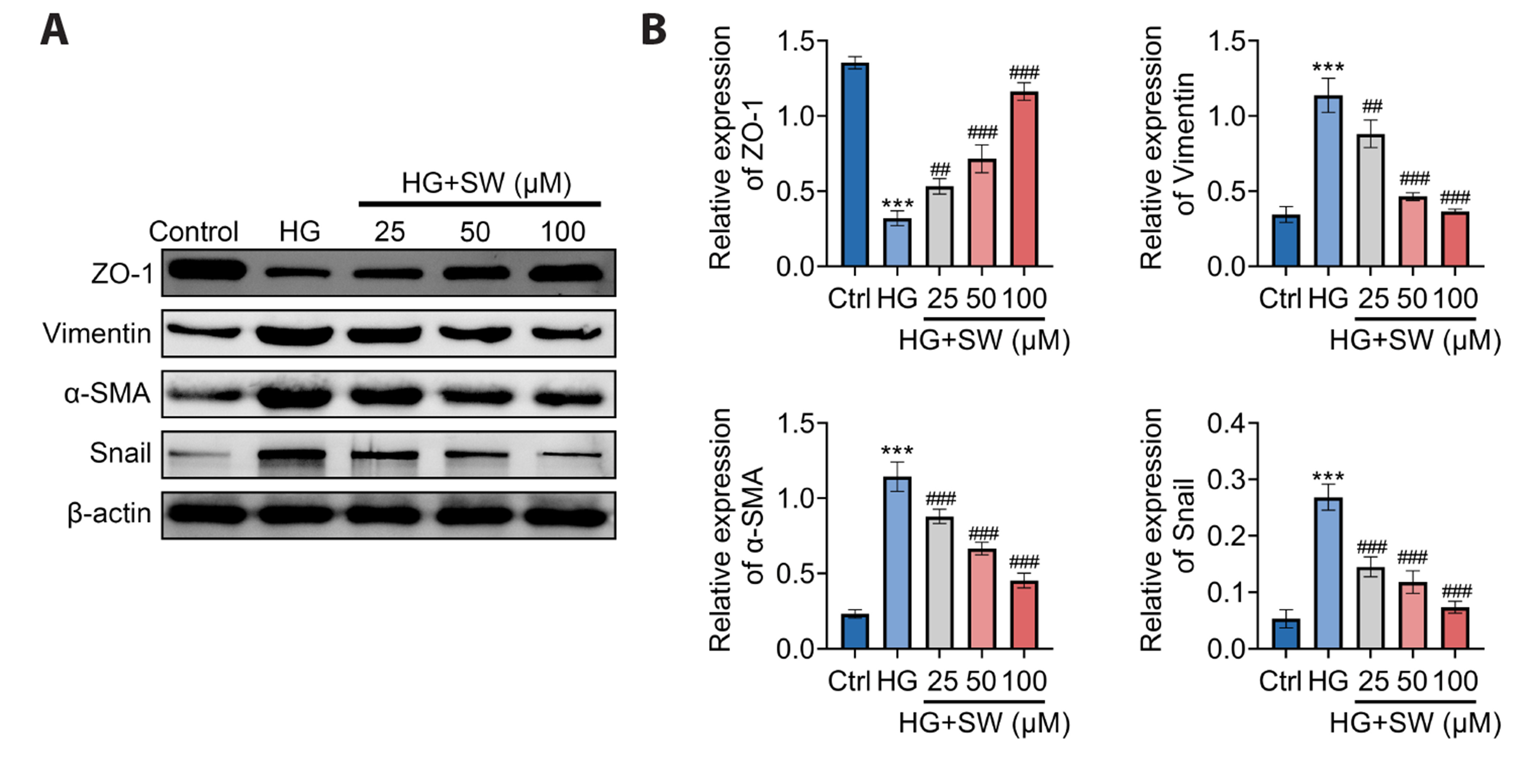
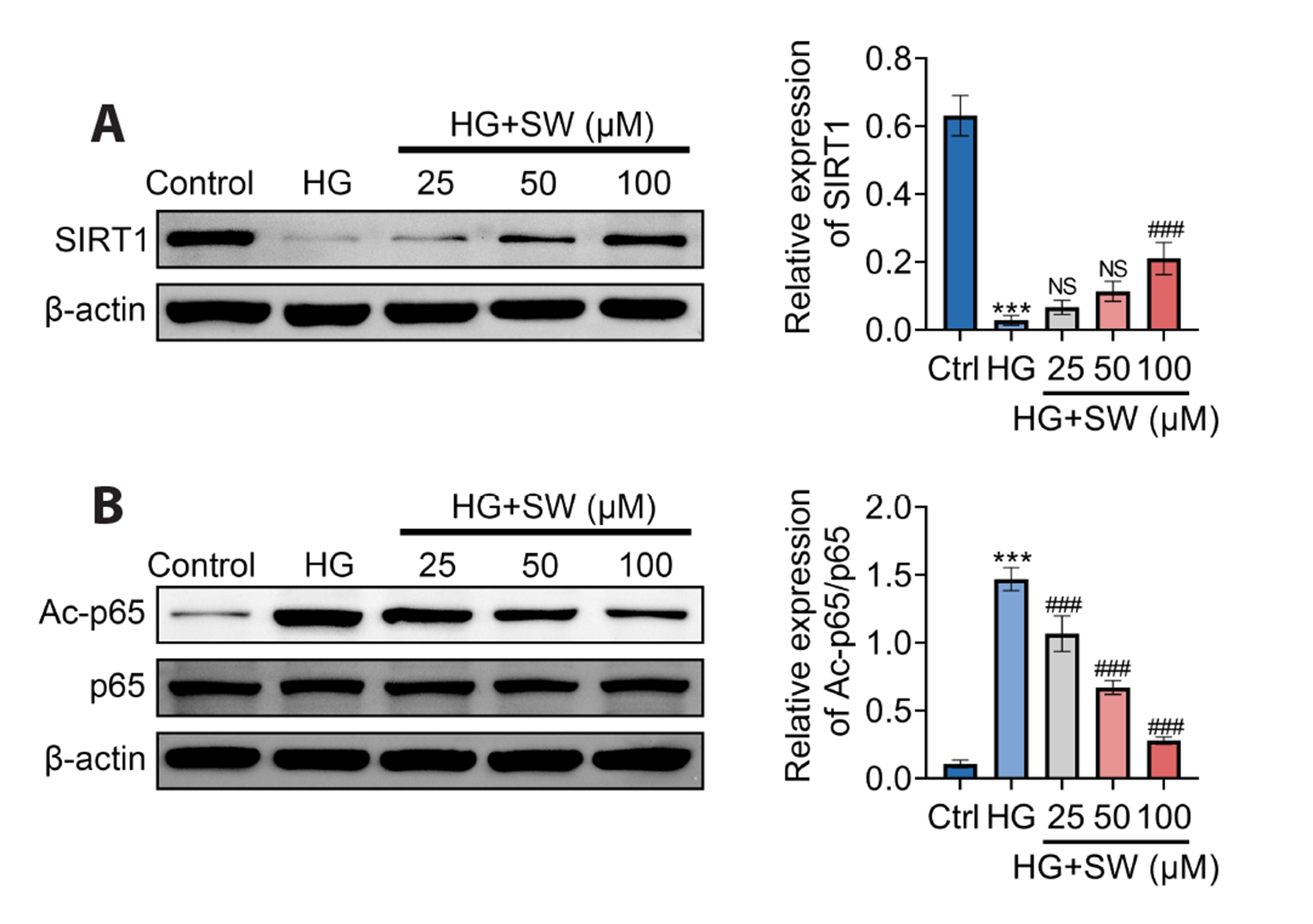
- Sweroside is a natural monoterpene derived from Swertia pseudochinensis Hara. Recently, studies have shown that sweroside exhibits a variety of biological activities, such as anti-inflammatory, antioxidant, and hypoglycemic effects. However, its role and mechanisms in high glucose (HG)-induced renal injury remain unclear. Herein, we established a renal injury model in vitro by inducing human renal tubular epithelial cell (HK-2 cells) injury by HG. Then, the effects of sweroside on HK-2 cell activity, inflammation, reactive oxygen species (ROS) production, and epithelial mesenchymal transition (EMT) were observed. As a result, sweroside treatment ameliorated the viability, inhibited the secretion of inflammatory cytokines (TNF-α, IL-1β, and VCAM-1), reduced the generation of ROS, and inhibited EMT in HK-2 cells. Moreover, the protein expression of SIRT1 was increased and the acetylation of p65 NFkB was decreased in HK-2 cells with sweroside treatment. More importantly, EX527, an inhibitor of SIRT1, that inactivated SIRT1, abolished the improvement effects of sweroside on HK-2 cells. Our findings suggested that sweroside may mitigate HGcaused injury in HK-2 cells by promoting SIRT1-mediated deacetylation of p65 NF-kB.
Keyword
Figure
Reference
-
1. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. 2017; Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 317:2515–2523. DOI: 10.1001/jama.2017.7596. PMID: 28655017. PMCID: PMC5815077.
Article2. Vahid H, Rakhshandeh H, Ghorbani A. 2017; Antidiabetic properties of Capparis spinosa L. and its components. Biomed Pharmacother. 92:293–302. DOI: 10.1016/j.biopha.2017.05.082. PMID: 28551550.
Article3. Chi K, Geng X, Liu C, Cai G, Hong Q. 2020; Research progress on the role of inflammasomes in kidney disease. Mediators Inflamm. 2020:8032797. DOI: 10.1155/2020/8032797. PMID: 32410864. PMCID: PMC7204206. PMID: 310b2e9c3709471997608d226f173bf7.
Article4. Masood S, Rehman AU, Bashir S, El Shazly M, Imran M, Khalil P, Ifthikar F, Jaffar HM, Khursheed T. 2021; Investigation of the anti-hyperglycemic and antioxidant effects of wheat bread supplemented with onion peel extract and onion powder in diabetic rats. J Diabetes Metab Disord. 20:485–495. DOI: 10.1007/s40200-021-00770-x. PMID: 34222073. PMCID: PMC8212200.
Article5. Samsu N. 2021; Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021:1497449. DOI: 10.1155/2021/1497449. PMID: 34307650. PMCID: PMC8285185.
Article6. Zheng C, Huang L, Luo W, Yu W, Hu X, Guan X, Cai Y, Zou C, Yin H, Xu Z, Liang G, Wang Y. 2019; Inhibition of STAT3 in tubular epithelial cells prevents kidney fibrosis and nephropathy in STZ-induced diabetic mice. Cell Death Dis. 10:848. DOI: 10.1038/s41419-019-2085-0. PMID: 31699972. PMCID: PMC6838321.
Article7. Liu L, Bai F, Song H, Xiao R, Wang Y, Yang H, Ren X, Li S, Gao L, Ma C, Yang X, Liang X. 2022; Upregulation of TIPE1 in tubular epithelial cell aggravates diabetic nephropathy by disrupting PHB2 mediated mitophagy. Redox Biol. 50:102260. Erratum in: Redox Biol. 2022;52:102302. DOI: 10.1016/j.redox.2022.102302. PMID: 35365434. PMCID: PMC9108084.
Article8. Gong J, Yang F, Yang Q, Tang X, Shu F, Xu L, Wang Z, Yang L. 2020; Sweroside ameliorated carbon tetrachloride (CCl4)-induced liver fibrosis through FXR-miR-29a signaling pathway. J Nat Med. 74:17–25. DOI: 10.1007/s11418-019-01334-3. PMID: 31280460.
Article9. Nuntawong P, Horikawa T, Tanaka H, Morimoto S, Sakamoto S. 2022; Activated carbon-based immunochromatographic strip test for the rapid qualitative analysis of swertiamarin and sweroside. J AOAC Int. 105:1460–1467. DOI: 10.1093/jaoacint/qsac054. PMID: 35521980.
Article10. Wang J, Cai X, Ma R, Lei D, Pan X, Wang F. 2021; Anti-inflammatory effects of sweroside on LPS-induced ALI in mice via activating SIRT1. Inflammation. 44:1961–1968. DOI: 10.1007/s10753-021-01473-4. PMID: 33913051.
Article11. Li J, Zhao C, Zhu Q, Wang Y, Li G, Li X, Li Y, Wu N, Ma C. 2021; Sweroside protects against myocardial ischemia-reperfusion injury by inhibiting oxidative stress and pyroptosis partially via modulation of the Keap1/Nrf2 axis. Front Cardiovasc Med. 8:650368. DOI: 10.3389/fcvm.2021.650368. PMID: 33816579. PMCID: PMC8017130. PMID: dc69b42000b5434ba587be0b47535f43.
Article12. Wang R, Dong Z, Lan X, Liao Z, Chen M. 2019; Sweroside alleviated LPS-induced inflammation via SIRT1 mediating NF-κB and FOXO1 signaling pathways in RAW264.7 cells. Molecules. 24:872. DOI: 10.3390/molecules24050872. PMID: 30823686. PMCID: PMC6429084. PMID: d5f4aae37d7548d18d8ae4f76f5f4d0d.
Article13. Yang G, Jang JH, Kim SW, Han SH, Ma KH, Jang JK, Kang HC, Cho YY, Lee HS, Lee JY. 2020; Sweroside prevents non-alcoholic steatohepatitis by suppressing activation of the NLRP3 inflammasome. Int J Mol Sci. 21:2790. DOI: 10.3390/ijms21082790. PMID: 32316419. PMCID: PMC7216241. PMID: 8a50a65db41243f9a4ea0f916a0365c6.
Article14. Su WY, Li Y, Chen X, Li X, Wei H, Liu Z, Shen Q, Chen C, Wang YP, Li W. 2021; Ginsenoside Rh1 improves type 2 diabetic nephropathy through AMPK/PI3K/Akt-mediated inflammation and apoptosis signaling pathway. Am J Chin Med. 49:1215–1233. DOI: 10.1142/S0192415X21500580. PMID: 34049473.
Article15. Darenskaya MA, Kolesnikova LI, Kolesnikov SI. 2021; Oxidative stress: pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull Exp Biol Med. 171:179–189. DOI: 10.1007/s10517-021-05191-7. PMID: 34173093. PMCID: PMC8233182.
Article16. Østergaard JA, Cooper ME, Jandeleit-Dahm KAM. 2020; Targeting oxidative stress and anti-oxidant defence in diabetic kidney disease. J Nephrol. 33:917–929. DOI: 10.1007/s40620-020-00749-6. PMID: 32447617.
Article17. Jiang WJ, Xu CT, Du CL, Dong JH, Xu SB, Hu BF, Feng R, Zang DD, Meng XM, Huang C, Li J, Ma TT. 2022; Tubular epithelial cell-to-macrophage communication forms a negative feedback loop via extracellular vesicle transfer to promote renal inflammation and apoptosis in diabetic nephropathy. Theranostics. 12:324–339. DOI: 10.7150/thno.63735. PMID: 34987648. PMCID: PMC8690920.
Article18. Xu H, Wang M, Li Y, Shi M, Wang Z, Cao C, Hong Y, Hu B, Zhu H, Zhao Z, Chu X, Zhu F, Deng X, Wu J, Zhao F, Guo J, Wang Y, Pei G, Zhu F, Wang X, et al. 2022; Blocking connexin 43 and its promotion of ATP release from renal tubular epithelial cells ameliorates renal fibrosis. Cell Death Dis. 13:511. DOI: 10.1038/s41419-022-04910-w. PMID: 35641484. PMCID: PMC9156700. PMID: 6f6ee1751e934bffab9d0808415a38ad.
Article19. Sun Y, Cai H, Ge J, Shao F, Huang Z, Ding Z, Dong L, Chen J, Zhang J, Zang Y. 2022; Tubule-derived INHBB promotes interstitial fibroblast activation and renal fibrosis. J Pathol. 256:25–37. DOI: 10.1002/path.5798. PMID: 34543458.
Article20. Mu L, Chen N, Chen Y, Yang Z, Zhou H, Song S, Shi Y. 2022; Blocking REDD1/TXNIP complex ameliorates HG-induced renal tubular epithelial cell apoptosis and EMT through repressing oxidative stress. Int J Endocrinol. 2022:6073911. DOI: 10.1155/2022/6073911. PMID: 36186658. PMCID: PMC9519289. PMID: fa5785edd79349fba71e38cee71f5761.
Article21. Gong EY, Jo HA, Park SH, Cha DR, Hur DY, Han SY. 2020; VSIG4 induces epithelial-mesenchymal transition of renal tubular cells under high-glucose conditions. Life (Basel). 10:354. DOI: 10.3390/life10120354. PMID: 33348749. PMCID: PMC7766063. PMID: 2b8591792cf54a8996235a2f6d1b95e0.
Article22. Wang WW, Liu YL, Wang MZ, Li H, Liu BH, Tu Y, Yuan CC, Fang QJ, Chen JX, Wang J, Fu Y, Wan ZY, Wan YG, Wu W. 2021; Inhibition of renal tubular epithelial mesenchymal transition and endoplasmic reticulum stress-induced apoptosis with Shenkang injection attenuates diabetic tubulopathy. Front Pharmacol. 12:662706. DOI: 10.3389/fphar.2021.662706. PMID: 34408650. PMCID: PMC8367077. PMID: 6e5a17a3b367498cb8b8c6b3e2d83cd2.
Article23. Zang L, Gao F, Huang A, Zhang Y, Luo Y, Chen L, Mao N. 2022; Icariin inhibits epithelial mesenchymal transition of renal tubular epithelial cells via regulating the miR-122-5p/FOXP2 axis in diabetic nephropathy rats. J Pharmacol Sci. 148:204–213. DOI: 10.1016/j.jphs.2021.10.002. PMID: 35063135.
Article24. Morigi M, Perico L, Benigni A. 2018; Sirtuins in renal health and disease. J Am Soc Nephrol. 29:1799–1809. DOI: 10.1681/ASN.2017111218. PMID: 29712732. PMCID: PMC6050939.
Article25. Yan J, Wang J, He JC, Zhong Y. 2022; Sirtuin 1 in chronic kidney disease and therapeutic potential of targeting Sirtuin 1. Front Endocrinol (Lausanne). 13:917773. DOI: 10.3389/fendo.2022.917773. PMID: 35795148. PMCID: PMC9251114. PMID: dd1232cc2b4042e495d9c1e72fc56a1b.
Article26. Qiu D, Song S, Wang Y, Bian Y, Wu M, Wu H, Shi Y, Duan H. 2022; NAD(P)H: quinone oxidoreductase 1 attenuates oxidative stress and apoptosis by regulating Sirt1 in diabetic nephropathy. J Transl Med. 20:44. DOI: 10.1186/s12967-021-03197-3. PMID: 35090502. PMCID: PMC8796493. PMID: b38ee5a4e12d48039ab077a30b16484f.
Article27. Du L, Qian X, Li Y, Li XZ, He LL, Xu L, Liu YQ, Li CC, Ma P, Shu FL, Lu Q, Yin XX. 2021; Sirt1 inhibits renal tubular cell epithelial-mesenchymal transition through YY1 deacetylation in diabetic nephropathy. Acta Pharmacol Sin. 42:242–251. DOI: 10.1038/s41401-020-0450-2. PMID: 32555442. PMCID: PMC8027604.
Article28. Shen J, Dai Z, Li Y, Zhu H, Zhao L. 2022; TLR9 regulates NLRP3 inflammasome activation via the NF-kB signaling pathway in diabetic nephropathy. Diabetol Metab Syndr. 14:26. DOI: 10.1186/s13098-021-00780-y. PMID: 35120573. PMCID: PMC8815223. PMID: bda1900c32394c89a7ce7548fac756cd.
Article29. Tao Y, Lin Y, An L, Tao Y, Li Y, Han J, Hu W, Liu W. 2022; Knockdown of GPRC5B alleviates the high glucose-induced inflammation and extracellular matrix deposition of podocyte through inhibiting NF-κB pathway. Allergol Immunopathol (Madr). 50:142–146. DOI: 10.15586/aei.v50i2.566. PMID: 35257557.
Article30. Fu Y, Wang X, Zhang L, Ren Y, Hao L. 2022; Allograft inflammatory factor-1 enhances inflammation and oxidative stress via the NF-κB pathway in diabetic kidney disease. Biochem Biophys Res Commun. 614:63–69. DOI: 10.1016/j.bbrc.2022.04.089. PMID: 35569377.
Article31. Zhang Y, Chen X, Fan Y, Liu J, Yuan L. 2022; XCL1 aggravates diabetic nephropathy-mediated renal glomerular endothelial cell apoptosis and inflammatory response via regulating p53/nuclear factor-kappa B pathway. Nephron. 146:84–98. DOI: 10.1159/000518172. PMID: 34518457.
Article32. Liu Y, Liu W, Zhang Z, Hu Y, Zhang X, Sun Y, Lei Q, Sun D, Liu T, Fan Y, Li H, Ding W, Fang J. 2021; Yishen capsule promotes podocyte autophagy through regulating SIRT1/NF-κB signaling pathway to improve diabetic nephropathy. Ren Fail. 43:128–140. DOI: 10.1080/0886022X.2020.1869043. PMID: 33427556. PMCID: PMC7808384. PMID: 8893498db4184214915f16bd0f2dddbe.
Article33. Sun HJ, Xiong SP, Cao X, Cao L, Zhu MY, Wu ZY, Bian JS. 2021; Polysulfide-mediated sulfhydration of SIRT1 prevents diabetic nephropathy by suppressing phosphorylation and acetylation of p65 NF-κB and STAT3. Redox Biol. 38:101813. DOI: 10.1016/j.redox.2020.101813. PMID: 33279869. PMCID: PMC7718489.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells
- MIR210HG Aggravates Sepsis-Induced Inflammatory Response of Proximal Tubular Epithelial Cell via the NF-κB Signaling Pathway
- The role of discoid domain receptor 1 on renal tubular epithelial pyroptosis in diabetic nephropathy
- Aurantio-obtusin exerts an anti-inflammatory effect on acute kidney injury by inhibiting NF-κκB pathway
- Pyronaridine Inhibited MUC5AC Mucin Gene Expression by Regulation of Nuclear Factor Kappa B Signaling Pathway in Human Pulmonary Mucoepidermoid Cells