Korean J Physiol Pharmacol.
2022 Nov;26(6):427-438. 10.4196/kjpp.2022.26.6.427.
The role of discoid domain receptor 1 on renal tubular epithelial pyroptosis in diabetic nephropathy
- Affiliations
-
- 1Department of Pharmacy, Lu'an Hospital Affiliated to Anhui Medical University, Lu’an People’s Hospital, Lu'an, Anhui 237005, China
- KMID: 2534519
- DOI: http://doi.org/10.4196/kjpp.2022.26.6.427
Abstract
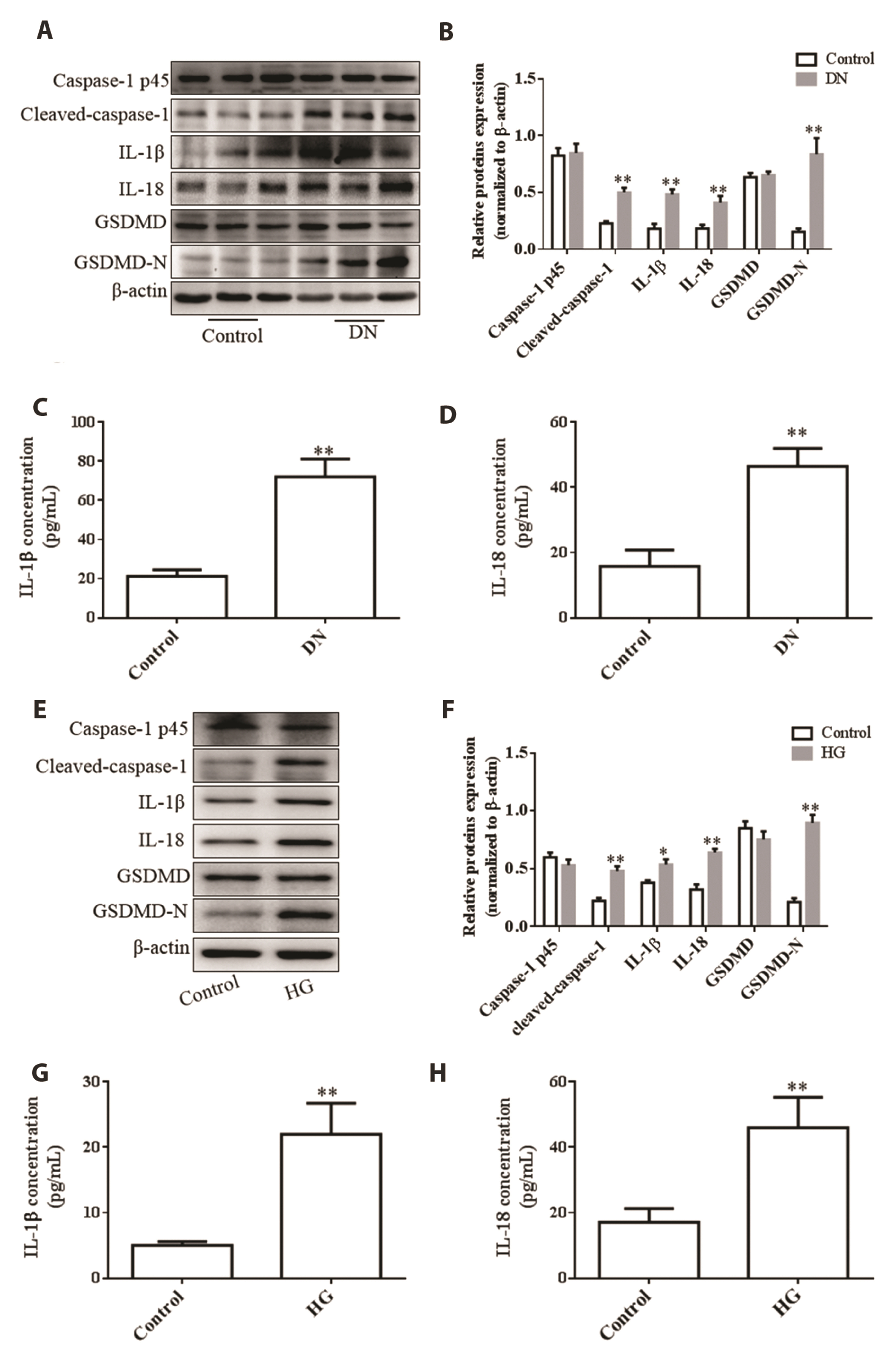
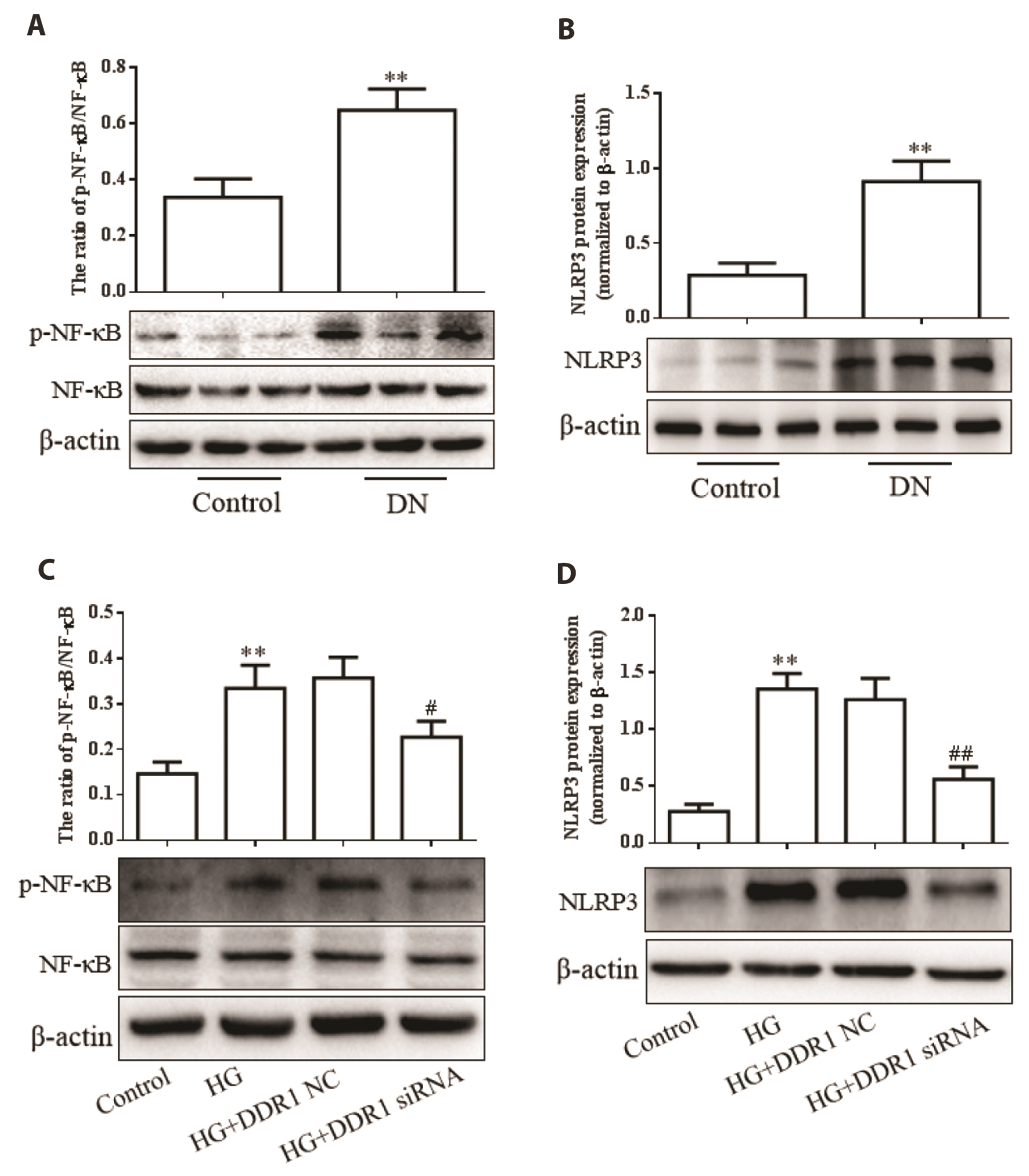
- Pyroptosis, a form of cell death associated with inflammation, is known to be involved in diabetic nephropathy (DN), and discoid domain receptor 1 (DDR1), an inflammatory regulatory protein, is reported to be associated with diabetes. However, the mechanism underlying DDR1 regulation and pyroptosis in DN remains unknown. We aimed to investigate the effect of DDR1 on renal tubular epithelial cell pyroptosis and the mechanism underlying DN. In this study, we used high glucose (HG)-treated HK-2 cells and rats with a single intraperitoneal injection of streptozotocin as DN models. Subsequently, the expression of pyroptosis-related proteins (cleaved caspase-1, GSDMD-N, Interleukin-1β [IL-1β], and interleukin-18 [IL-18]), DDR1, phosphorylated NF-κB (p-NF-κB), and NLR family pyrin domain-containing 3 (NLRP3) inflammasomes were determined through Western blotting. IL-1β and IL-18 levels were determined using ELISA. The rate of pyroptosis was assessed by propidium iodide (PI) staining. The results revealed upregulated expression of pyroptosisrelated proteins and increased concentration of IL-1β and IL-18, accompanied by DDR1, p-NF-κB, and NLRP3 upregulation in DN rat kidney tissues and HG-treated HK-2 cells. Moreover, DDR1 knockdown in the background of HG treatment resulted in inhibited expression of pyroptosis-related proteins and attenuation of IL-1β and IL-18 production and PI-positive cell frequency via the NF-κB/NLRP3 pathway in HK-2 cells. However, NLRP3 overexpression reversed the effect of DDR1 knockdown on pyroptosis. In conclusion, we demonstrated that DDR1 may be associated with pyroptosis, and DDR1 knockdown inhibited HG-induced renal tubular epithelial cell pyroptosis. The NF-κB/NLRP3 pathway is probably involved in the underlying mechanism of these findings.
Keyword
Figure
Cited by 1 articles
-
Experimental model and novel therapeutic targets for non-alcoholic fatty liver disease development
Yujin Jin, Kyung-Sun Heo
Korean J Physiol Pharmacol. 2023;27(4):299-310. doi: 10.4196/kjpp.2023.27.4.299.
Reference
-
1. Papadopoulou-Marketou N, Paschou SA, Marketos N, Adamidi S, Adamidis S, Kanaka-Gantenbein C. 2018; Diabetic nephropathy in type 1 diabetes. Minerva Med. 109:218–228. DOI: 10.23736/S0026-4806.17.05496-9. PMID: 29205998. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85048813564&origin=inward.
Article2. Pérez-Morales RE, Del Pino MD, Valdivielso JM, Ortiz A, Mora-Fernández C, Navarro-González JF. 2019; Inflammation in diabetic kidney disease. Nephron. 143:12–16. DOI: 10.1159/000493278. PMID: 30273931. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85054589616&origin=inward.
Article3. Wu L, Liu C, Chang DY, Zhan R, Sun J, Cui SH, Eddy S, Nair V, Tanner E, Brosius FC, Looker HC, Nelson RG, Kretzler M, Wang JC, Xu M, Ju W, Zhao MH, Chen M, Zheng L. 2021; Annexin A1 alleviates kidney injury by promoting the resolution of inflammation in diabetic nephropathy. Kidney Int. 100:107–121. Erratum in: Kidney Int. 2021;100:1349-1350. DOI: 10.1016/j.kint.2021.02.025. PMID: 33675846. PMCID: PMC8893600. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85104313122&origin=inward.
Article4. An X, Zhang Y, Cao Y, Chen J, Qin H, Yang L. 2020; Punicalagin protects diabetic nephropathy by inhibiting pyroptosis based on TXNIP/NLRP3 pathway. Nutrients. 12:1516. DOI: 10.3390/nu12051516. PMID: 32456088. PMCID: PMC7284711. PMID: bc435bbd1d34432b88658dab2e8ee2f6. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085209979&origin=inward.
Article5. Han J, Zuo Z, Shi X, Zhang Y, Peng Z, Xing Y, Pang X. 2021; Hirudin ameliorates diabetic nephropathy by inhibiting Gsdmd-mediated pyroptosis. Cell Biol Toxicol. doi: 10.1007/s10565-021-09622-z. [Epub ahead of print]. DOI: 10.1007/s10565-021-09622-z. PMID: 34212273. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85109299858&origin=inward.
Article6. Zhaolin Z, Guohua L, Shiyuan W, Zuo W. 2019; Role of pyroptosis in cardiovascular disease. Cell Prolif. 52:e12563. DOI: 10.1111/cpr.12563. PMID: 30525268. PMCID: PMC6496801. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85058158091&origin=inward.
Article7. Wu X, Zhang H, Qi W, Zhang Y, Li J, Li Z, Lin Y, Bai X, Liu X, Chen X, Yang H, Xu C, Zhang Y, Yang B. 2018; Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 9:171. DOI: 10.1038/s41419-017-0257-3. PMID: 29416034. PMCID: PMC5833729. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85041693664&origin=inward.
Article8. Burdette BE, Esparza AN, Zhu H, Wang S. 2021; Gasdermin D in pyroptosis. Acta Pharm Sin B. 11:2768–2782. DOI: 10.1016/j.apsb.2021.02.006. PMID: 34589396. PMCID: PMC8463274. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85111499098&origin=inward.
Article9. Xia S, Zhang Z, Magupalli VG, Pablo JL, Dong Y, Vora SM, Wang L, Fu TM, Jacobson MP, Greka A, Lieberman J, Ruan J, Wu H. 2021; Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 593:607–611. DOI: 10.1038/s41586-021-03478-3. PMID: 33883744. PMCID: PMC8588876. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85104775814&origin=inward.
Article10. Liu C, Zhuo H, Ye MY, Huang GX, Fan M, Huang XZ. 2020; LncRNA MALAT1 promoted high glucose-induced pyroptosis of renal tubular epithelial cell by sponging miR-30c targeting for NLRP3. Kaohsiung J Med Sci. 36:682–691. DOI: 10.1002/kjm2.12226. PMID: 32391974. PMID: 890320e4d2f24cafade258652fc96001. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85084455177&origin=inward.
Article11. Gu J, Huang W, Zhang W, Zhao T, Gao C, Gan W, Rao M, Chen Q, Guo M, Xu Y, Xu YH. 2019; Sodium butyrate alleviates high-glucose-induced renal glomerular endothelial cells damage via inhibiting pyroptosis. Int Immunopharmacol. 75:105832. DOI: 10.1016/j.intimp.2019.105832. PMID: 31473434. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071400890&origin=inward.
Article12. Zhou K, Zhang J, Liu C, Ou L, Wang F, Yu Y, Wang Y, Bai S. 2021; Sanziguben polysaccharides inhibit diabetic nephropathy through NF-κB-mediated anti-inflammation. Nutr Metab (Lond). 18:81. DOI: 10.1186/s12986-021-00601-z. PMID: 34493288. PMCID: PMC8425148. PMID: 4980afbe3f9444d59df7112f4e555ef3. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85114408320&origin=inward.
Article13. Zheng ZC, Zhu W, Lei L, Liu XQ, Wu YG. 2020; Wogonin ameliorates renal inflammation and fibrosis by inhibiting NF-κB and TGF-β1/Smad3 signaling pathways in diabetic nephropathy. Drug Des Devel Ther. 14:4135–4148. DOI: 10.2147/DDDT.S274256. PMID: 33116403. PMCID: PMC7549498. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85092398377&origin=inward.14. Wu M, Yang Z, Zhang C, Shi Y, Han W, Song S, Mu L, Du C, Shi Y. 2021; Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism. 118:154748. DOI: 10.1016/j.metabol.2021.154748. PMID: 33675822. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85102270436&origin=inward.
Article15. Hou Y, Lin S, Qiu J, Sun W, Dong M, Xiang Y, Wang L, Du P. 2020; NLRP3 inflammasome negatively regulates podocyte autophagy in diabetic nephropathy. Biochem Biophys Res Commun. 521:791–798. DOI: 10.1016/j.bbrc.2019.10.194. PMID: 31703838. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85075460463&origin=inward.
Article16. El-Sisi AEE, Sokar SS, Shebl AM, Mohamed DZ, Abu-Risha SE. 2021; Octreotide and melatonin alleviate inflammasome-induced pyroptosis through inhibition of TLR4-NF-κB-NLRP3 pathway in hepatic ischemia/reperfusion injury. Toxicol Appl Pharmacol. 410:115340. DOI: 10.1016/j.taap.2020.115340. PMID: 33264646. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85097207719&origin=inward.
Article17. Chen X, Liu G, Yuan Y, Wu G, Wang S, Yuan L. 2019; NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis. 10:906. DOI: 10.1038/s41419-019-2157-1. PMID: 31787755. PMCID: PMC6885517. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85075779323&origin=inward.
Article18. Li F, Xu D, Hou K, Gou X, Lv N, Fang W, Li Y. 2021; Pretreatment of indobufen and aspirin and their combinations with clopidogrel or ticagrelor alleviates inflammasome mediated pyroptosis via inhibiting NF-κB/NLRP3 pathway in ischemic stroke. J Neuroimmune Pharmacol. 16:835–853. DOI: 10.1007/s11481-020-09978-9. PMID: 33512659. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85099983829&origin=inward.
Article19. Wang Y, Zhu X, Yuan S, Wen S, Liu X, Wang C, Qu Z, Li J, Liu H, Sun L, Liu F. 2019; TLR4/NF-κB signaling induces GSDMD-related pyroptosis in tubular cells in diabetic kidney disease. Front Endocrinol (Lausanne). 10:603. DOI: 10.3389/fendo.2019.00603. PMID: 31608008. PMCID: PMC6761221. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85072989012&origin=inward.
Article20. Yeh YC, Lin HH, Tang MJ. 2019; Dichotomy of the function of DDR1 in cells and disease progression. Biochim Biophys Acta Mol Cell Res. 1866:118473. DOI: 10.1016/j.bbamcr.2019.04.003. PMID: 30954568. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064591684&origin=inward.
Article21. Leitinger B. 2014; Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol. 310:39–87. DOI: 10.1016/B978-0-12-800180-6.00002-5. PMID: 24725424. PMCID: PMC4021107. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84898029568&origin=inward.
Article22. Yan L, Xie X, Niu BX, Wu MT, Tong WQ, He SY, Huang CY, Zhao WC, Li G, Li NS, Jiang JL. 2021; Involvement of miR-199a-3p/DDR1 in vascular endothelial cell senescence in diabetes. Eur J Pharmacol. 908:174317. DOI: 10.1016/j.ejphar.2021.174317. PMID: 34270989. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85110319625&origin=inward.
Article23. Kerroch M, Guerrot D, Vandermeersch S, Placier S, Mesnard L, Jouanneau C, Rondeau E, Ronco P, Boffa JJ, Chatziantoniou C, Dussaule JC. 2012; Genetic inhibition of discoidin domain receptor 1 protects mice against crescentic glomerulonephritis. FASEB J. 26:4079–4091. DOI: 10.1096/fj.11-194902. PMID: 22751008. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84868089019&origin=inward.
Article24. Shen B, Wang F, Zhou Y, Li T, He C, Zhao W. 2021; Ginsenoside Rh2 inhibits renal fibrosis and renal cell apoptosis in rats with diabetic nephropathy by downregulating discoid domain receptor 1. Nan Fang Yi Ke Da Xue Xue Bao. 41:1107–1113. Chinese. DOI: 10.1096/fj.11-194902. PMID: 34308864. PMCID: PMC8329676. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84868089019&origin=inward.25. Kwon G, Uddin MJ, Lee G, Jiang S, Cho A, Lee JH, Lee SR, Bae YS, Moon SH, Lee SJ, Cha DR, Ha H. 2017; A novel pan-Nox inhibitor, APX-115, protects kidney injury in streptozotocin-induced diabetic mice: possible role of peroxisomal and mitochondrial biogenesis. Oncotarget. 8:74217–74232. DOI: 10.18632/oncotarget.18540. PMID: 29088780. PMCID: PMC5650335. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030100536&origin=inward.
Article26. Koye DN, Shaw JE, Reid CM, Atkins RC, Reutens AT, Magliano DJ. 2017; Incidence of chronic kidney disease among people with diabetes: a systematic review of observational studies. Diabet Med. 34:887–901. DOI: 10.1111/dme.13324. PMID: 28164387. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85014785989&origin=inward.
Article27. Li P, Dong XR, Zhang B, Zhang XT, Liu JZ, Ma DS, Ma L. 2021; Molecular mechanism and therapeutic targeting of necrosis, apoptosis, pyroptosis, and autophagy in cardiovascular disease. Chin Med J (Engl). 134:2647–2655. DOI: 10.1097/CM9.0000000000001772. PMID: 34608069. PMCID: PMC8631411. PMID: 1188f001c57f4b9393944cbbadb63a68. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85120690324&origin=inward.
Article28. Wang Q, Wu J, Zeng Y, Chen K, Wang C, Yang S, Sun N, Chen H, Duan K, Zeng G. 2020; Pyroptosis: a pro-inflammatory type of cell death in cardiovascular disease. Clin Chim Acta. 510:62–72. DOI: 10.1016/j.cca.2020.06.044. PMID: 32622968. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85087889542&origin=inward.
Article29. Zhu W, Li YY, Zeng HX, Liu XQ, Sun YT, Jiang L, Xia LL, Wu YG. 2021; Carnosine alleviates podocyte injury in diabetic nephropathy by targeting caspase-1-mediated pyroptosis. Int Immunopharmacol. 101(Pt B):108236. DOI: 10.1016/j.intimp.2021.108236. PMID: 34653727. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85116865081&origin=inward.
Article30. Zhan JF, Huang HW, Huang C, Hu LL, Xu WW. 2020; Long non-coding RNA NEAT1 regulates pyroptosis in diabetic nephropathy via mediating the miR-34c/NLRP3 axis. Kidney Blood Press Res. 45:589–602. DOI: 10.1159/000508372. PMID: 32721950. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089129905&origin=inward.
Article31. Zhao W, He C, Wang F. 2020; Screening potential Chinese materia medica and their monomers for treatment diabetic nephropathy based on caspase-1-mediated pyroptosis. Nan Fang Yi Ke Da Xue Xue Bao. 40:1280–1287. Chinese. DOI: 10.12122/j.issn.1673-4254.2020.09.09. PMID: 32990240. PMCID: PMC7544587. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85092121196&origin=inward.32. Zhu M, Xing D, Lu Z, Fan Y, Hou W, Dong H, Xiong L, Dong H. 2015; DDR1 may play a key role in destruction of the blood-brain barrier after cerebral ischemia-reperfusion. Neurosci Res. 96:14–19. DOI: 10.1016/j.neures.2015.01.004. PMID: 25630038. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84929944185&origin=inward.
Article33. Lino M, Wan MH, Rocca AS, Ngai D, Shobeiri N, Hou G, Ge C, Franceschi RT, Bendeck MP. 2018; Diabetic vascular calcification mediated by the collagen receptor discoidin domain receptor 1 via the phosphoinositide 3-kinase/Akt/runt-related transcription factor 2 signaling axis. Arterioscler Thromb Vasc Biol. 38:1878–1889. DOI: 10.1161/ATVBAHA.118.311238. PMID: 29930002. PMCID: PMC6441610. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85055597507&origin=inward.
Article34. Malaguarnera R, Nicolosi ML, Sacco A, Morcavallo A, Vella V, Voci C, Spatuzza M, Xu SQ, Iozzo RV, Vigneri R, Morrione A, Belfiore A. 2015; Novel cross talk between IGF-IR and DDR1 regulates IGF-IR trafficking, signaling and biological responses. Oncotarget. 6:16084–16105. DOI: 10.18632/oncotarget.3177. PMID: 25840417. PMCID: PMC4599258. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84937836761&origin=inward.
Article35. Matà R, Palladino C, Nicolosi ML, Lo Presti AR, Malaguarnera R, Ragusa M, Sciortino D, Morrione A, Maggiolini M, Vella V, Belfiore A. 2016; IGF-I induces upregulation of DDR1 collagen receptor in breast cancer cells by suppressing MIR-199a-5p through the PI3K/AKT pathway. Oncotarget. 7:7683–7700. DOI: 10.18632/oncotarget.6524. PMID: 26655502. PMCID: PMC4884947. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84958817630&origin=inward.
Article36. Borza CM, Pozzi A. 2014; Discoidin domain receptors in disease. Matrix Biol. 34:185–192. DOI: 10.1016/j.matbio.2013.12.002. PMID: 24361528. PMCID: PMC4019715. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84900340780&origin=inward.
Article37. Moll S, Yasui Y, Abed A, Murata T, Shimada H, Maeda A, Fukushima N, Kanamori M, Uhles S, Badi L, Cagarelli T, Formentini I, Drawnel F, Georges G, Bergauer T, Gasser R, Bonfil RD, Fridman R, Richter H, Funk J, et al. 2018; Selective pharmacological inhibition of DDR1 prevents experimentally-induced glomerulonephritis in prevention and therapeutic regime. J Transl Med. 16:148. DOI: 10.1186/s12967-018-1524-5. PMID: 29859097. PMCID: PMC5984769. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85047970806&origin=inward.
Article38. Franco C, Hou G, Ahmad PJ, Fu EY, Koh L, Vogel WF, Bendeck MP. 2008; Discoidin domain receptor 1 (ddr1) deletion decreases atherosclerosis by accelerating matrix accumulation and reducing inflammation in low-density lipoprotein receptor-deficient mice. Circ Res. 102:1202–1211. DOI: 10.1161/CIRCRESAHA.107.170662. PMID: 18451340. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=45149117999&origin=inward.
Article39. Hou G, Vogel W, Bendeck MP. 2001; The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 107:727–735. DOI: 10.1172/JCI10720. PMID: 11254672. PMCID: PMC208942. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035107205&origin=inward.
Article40. Donate-Correa J, Martín-Núñez E, Muros-de-Fuentes M, Mora-Fernández C, Navarro-González JF. 2015; Inflammatory cytokines in diabetic nephropathy. J Diabetes Res. 2015:948417. DOI: 10.1155/2015/948417. PMID: 25785280. PMCID: PMC4345080. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84924246974&origin=inward.
Article41. Dong WH, Chu QQ, Liu SQ, Deng DT, Xu Q. 2020; Isobavachalcone ameliorates diabetic nephropathy in rats by inhibiting the NF-κB pathway. J Food Biochem. 44:e13405. DOI: 10.1111/jfbc.13405. PMID: 32710574. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85088397599&origin=inward.
Article42. Kolati SR, Kasala ER, Bodduluru LN, Mahareddy JR, Uppulapu SK, Gogoi R, Barua CC, Lahkar M. 2015; BAY 11-7082 ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress and renal inflammation via NF-κB pathway. Environ Toxicol Pharmacol. 39:690–699. DOI: 10.1016/j.etap.2015.01.019. PMID: 25704036. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84923044693&origin=inward.
Article43. Yi H, Peng R, Zhang LY, Sun Y, Peng HM, Liu HD, Yu LJ, Li AL, Zhang YJ, Jiang WH, Zhang Z. 2017; LincRNA-Gm4419 knockdown ameliorates NF-κB/NLRP3 inflammasome-mediated inflammation in diabetic nephropathy. Cell Death Dis. 8:e2583. DOI: 10.1038/cddis.2016.451. PMID: 28151474. PMCID: PMC5386454. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85011578559&origin=inward.
Article44. Tang K, Su W, Huang C, Wu Y, Wu X, Lu H. 2021; Notoginsenoside R1 suppresses inflammatory response and the pyroptosis of nucleus pulposus cells via inactivating NF-κB/NLRP3 pathways. Int Immunopharmacol. 101(Pt B):107866. DOI: 10.1016/j.intimp.2021.107866. PMID: 34588155. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85115930373&origin=inward.
Article45. Shen J, Ma H, Wang C. 2021; Triptolide improves myocardial fibrosis in rats through inhibition of nuclear factor kappa B and NLR family pyrin domain containing 3 inflammasome pathway. Korean J Physiol Pharmacol. 25:533–543. DOI: 10.4196/kjpp.2021.25.6.533. PMID: 34697264. PMCID: PMC8552823. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85119139136&origin=inward.
Article46. He Y, Hara H, Núñez G. 2016; Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 41:1012–1021. DOI: 10.1016/j.tibs.2016.09.002. PMID: 27669650. PMCID: PMC5123939. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84995751273&origin=inward.
Article47. Sun Z, Ma Y, Chen F, Wang S, Chen B, Shi J. 2018; Artesunate ameliorates high glucose-induced rat glomerular mesangial cell injury by suppressing the TLR4/NF-κB/NLRP3 inflammasome pathway. Chem Biol Interact. 293:11–19. DOI: 10.1016/j.cbi.2018.07.011. PMID: 30031708. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85050088669&origin=inward.
Article48. Yang J, Zhou Y, Shi J. 2020; Cordycepin protects against acute pancreatitis by modulating NF-κB and NLRP3 inflammasome activation via AMPK. Life Sci. 251:117645. DOI: 10.1016/j.lfs.2020.117645. PMID: 32268154. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082876564&origin=inward.
Article49. Chen X, Han R, Hao P, Wang L, Liu M, Jin M, Kong D, Li X. 2018; Nepetin inhibits IL-1β induced inflammation via NF-κB and MAPKs signaling pathways in ARPE-19 cells. Biomed Pharmacother. 101:87–93. DOI: 10.1016/j.biopha.2018.02.054. PMID: 29477475. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85042376888&origin=inward.
Article50. Peng L, Wen L, Shi QF, Gao F, Huang B, Meng J, Hu CP, Wang CM. 2020; Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 11:978. DOI: 10.1038/s41419-020-03178-2. PMID: 33188176. PMCID: PMC7666141. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85095939586&origin=inward.
Article51. Matsuyama W, Wang L, Farrar WL, Faure M, Yoshimura T. 2004; Activation of discoidin domain receptor 1 isoform b with collagen up-regulates chemokine production in human macrophages: role of p38 mitogen-activated protein kinase and NF-kappa B. J Immunol. 172:2332–2340. DOI: 10.4049/jimmunol.172.4.2332. PMID: 14764702. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0842278630&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High glucose stimulates the expression of erythropoietin in rat glomerular epithelial cells
- Recent Updates on Diabetic Nephropathy
- Pathophysiology of Diabetic Nephropathy
- Observation of neutrophil extracellular traps in the development of diabetic nephropathy using diabetic murine models
- The Epidemiology of Diabetic Nephropathy