Korean J Physiol Pharmacol.
2016 Nov;20(6):613-619. 10.4196/kjpp.2016.20.6.613.
Insulin-like growth factor-1 improves diabetic cardiomyopathy through antioxidative and anti-inflammatory processes along with modulation of Akt/GSK-3β signaling in rats
- Affiliations
-
- 1Center of Morphological Experiment, Medical College of Yanbian University, Yanji 133000, Jilin Province, China. dyxu@ybu.edu.cn
- KMID: 2364265
- DOI: http://doi.org/10.4196/kjpp.2016.20.6.613
Abstract
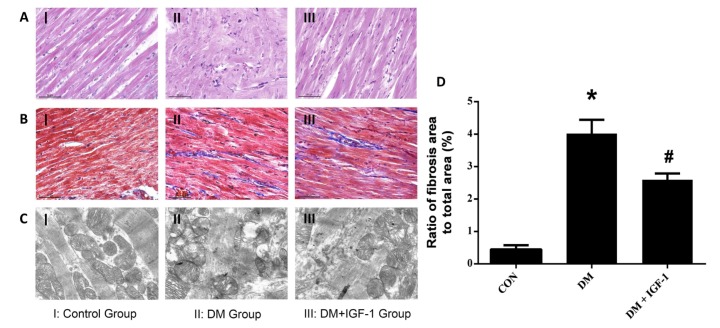
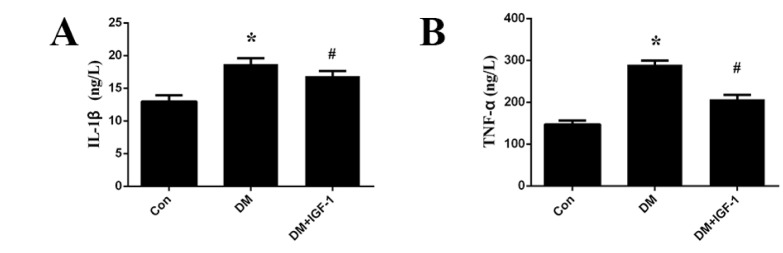
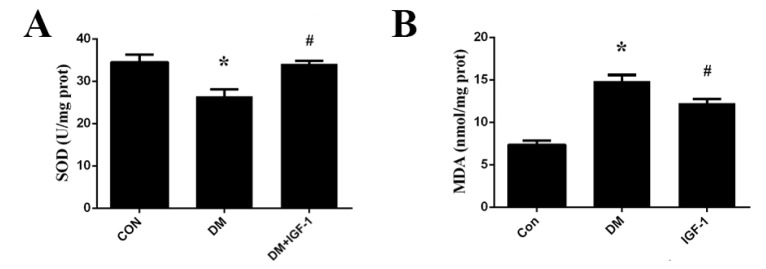
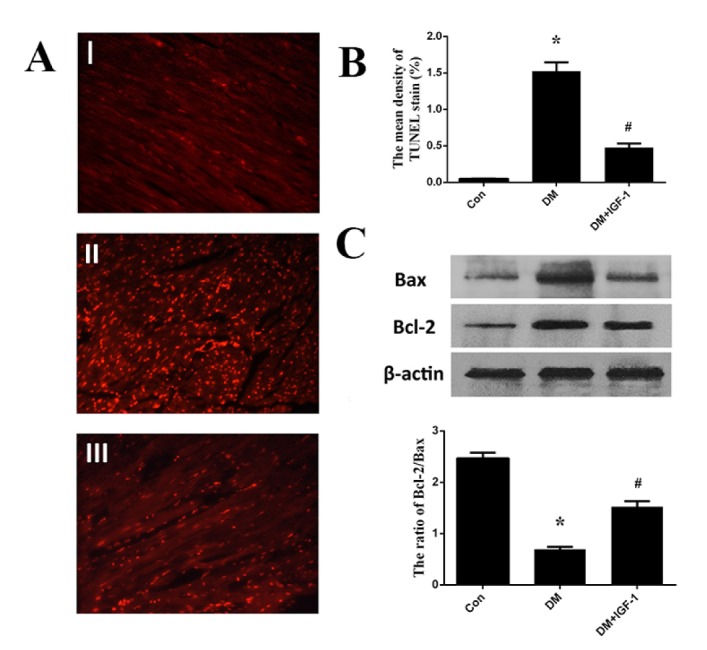
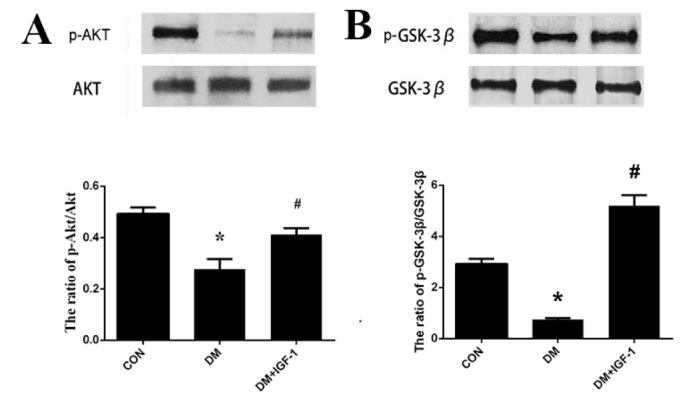
- Diabetic cardiomyopathy (DCM), a serious complication of diabetes mellitus, is associated with changes in myocardial structure and function. This study sought to explore the ability of insulin-like growth factor-1 (IGF-1) to modulate DCM and its related mechanisms. Twenty-four male Wistar rats were injected with streptozotocin (STZ, 60 mg/kg) to mimic diabetes mellitus. Myocardial fibrosis and apoptosis were evaluated by histopathologic analyses, and relevant proteins were analyzed by Western blotting. Inflammatory factors were assessed by ELISA. Markers of oxidative stress were tested by colorimetric analysis. Rats with DCM displayed decreased body weight, metabolic abnormalities, elevated apoptosis (as assessed by the bcl-2/bax ratio and TUNEL assays), increased fibrosis, increased markers of oxidative stress (MDA and SOD) and inflammatory factors (TNF-α and IL-1β), and decreased phosphorylation of Akt and glycogen synthase kinase (GSK-3β). IGF-1 treatment, however, attenuated the metabolic abnormalities and myocardial apoptosis, interstitial fibrosis, oxidative stress and inflammation seen in diabetic rats, while also increasing the phosphorylation levels of Akt and GSK-3β. These findings suggest that IGF-1 ameliorates the pathophysiological progress of DCM along with an activation of the Akt/GSK-3β signaling pathway. Our findings suggest that IGF-1 could be a potential therapeutic choice for controlling DCM.
MeSH Terms
-
Animals
Apoptosis
Blotting, Western
Body Weight
Diabetes Mellitus
Diabetic Cardiomyopathies*
Enzyme-Linked Immunosorbent Assay
Fibrosis
Glycogen Synthase Kinases
Humans
In Situ Nick-End Labeling
Inflammation
Insulin-Like Growth Factor I
Male
Oxidative Stress
Phosphorylation
Rats*
Rats, Wistar
Streptozocin
Glycogen Synthase Kinases
Insulin-Like Growth Factor I
Streptozocin
Figure
Reference
-
1. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009; 32:1335–1343. PMID: 19564476.
Article2. Huynh K, McMullen JR, Julius TL, Tan JW, Love JE, Cemerlang N, Kiriazis H, Du XJ, Ritchie RH. Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy. Diabetes. 2010; 59:1512–1520. PMID: 20215428.
Article3. Jiang LH, Yuan XL, Yang NY, Ren L, Zhao FM, Luo BX, Bian YY, Xu JY, Lu DX, Zheng YY, Zhang CJ, Diao YM, Xia BM, Chen G. Daucosterol protects neurons against oxygen-glucose deprivation/reperfusion-mediated injury by activating IGF1 signaling pathway. J Steroid Biochem Mol Biol. 2015; 152:45–52. PMID: 25864625.
Article4. Setshedi M, Longato L, Petersen DR, Ronis M, Chen WC, Wands JR, de la Monte SM. Limited therapeutic effect of N-acetylcysteine on hepatic insulin resistance in an experimental model of alcohol-induced steatohepatitis. Alcohol Clin Exp Res. 2011; 35:2139–2151. PMID: 21790669.
Article5. Shen Y, Qin J, Bu P. Pathways involved in interleukin-1beta-mediated murine cardiomyocyte apoptosis. Tex Heart Inst J. 2015; 42:109–116. PMID: 25873819.6. Norby FL, Wold LE, Duan J, Hintz KK, Ren J. IGF-I attenuates diabetes-induced cardiac contractile dysfunction in ventricular myocytes. Am J Physiol Endocrinol Metab. 2002; 283:E658–E666. PMID: 12217882.7. Han HJ, Kang CW, Park SH. Tissue-specific regulation of insulin-like growth factors and insulin-like growth factor binding proteins in male diabetic rats in vivo and in vitro. Clin Exp Pharmacol Physiol. 2006; 33:1172–1179. PMID: 17184497.
Article8. Ren J, Duan J, Thomas DP, Yang X, Sreejayan N, Sowers JR, Leri A, Kajstura J, Gao F, Anversa P. IGF-I alleviates diabetes-induced RhoA activation, eNOS uncoupling, and myocardial dysfunction. Am J Physiol Regul Integr Comp Physiol. 2008; 294:R793–R802. PMID: 18199585.
Article9. Kim MS, Lee DY. Serum insulin-like growth factor-binding protein-3 level correlated with glycemic control and lipid profiles in children and adolescents with type 1 diabetes. J Pediatr Endocrinol Metab. 2014; 27:857–861. PMID: 24825082.
Article10. Li J, Lin J, Song Y, Xiang L, Wu Z. Effects of insulin-like growth factor-1 on the myocardium in diabetic rats. Zhonghua Yi Xue Za Zhi. 2014; 94:3329–3333. PMID: 25622634.11. Kim MS, Lee DY. Insulin-like growth factor (IGF)-I and IGF binding proteins axis in diabetes mellitus. Ann Pediatr Endocrinol Metab. 2015; 20:69–73. PMID: 26191509.
Article12. Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P. IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes. 2001; 50:1414–1424. PMID: 11375343.
Article13. Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997; 275:661–665. PMID: 9005851.
Article14. Sanchez-Calderon H, Milo M, Leon Y, Varela-Nieto I. A network of growth and transcription factors controls neuronal differentation and survival in the developing ear. Int J Dev Biol. 2007; 51:557–570. PMID: 17891717.
Article15. Magariños M, Aburto MR, Sánchez-Calderón H, Muñoz-Agudo C, Rapp UR, Varela-Nieto I. RAF kinase activity regulates neuroepithelial cell proliferation and neuronal progenitor cell differentiation during early inner ear development. PLoS One. 2010; 5:e14435. PMID: 21203386.
Article16. Wang Y, Feng W, Xue W, Tan Y, Hein DW, Li XK, Cai L. Inactivation of GSK-3beta by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes. 2009; 58:1391–1402. PMID: 19324938.17. Yu W, Zha W, Ke Z, Min Q, Li C2, Sun H, Liu C. Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway. J Diabetes Res. 2016; 2016:4158591. PMID: 26989696.
Article18. Ganda OP, Rossini AA, Like AA. Studies on streptozotocin diabetes. Diabetes. 1976; 25:595–603. PMID: 132382.
Article19. Tarquini R, Lazzeri C, Pala L, Rotella CM, Gensini GF. The diabetic cardiomyopathy. Acta Diabetol. 2011; 48:173–181. PMID: 20198391.
Article20. Liu Q, Wang S, Cai L. Diabetic cardiomyopathy and its mechanisms: Role of oxidative stress and damage. J Diabetes Investig. 2014; 5:623–634.
Article21. Shan YX, Yang TL, Mestril R, Wang PH. Hsp10 and Hsp60 suppress ubiquitination of insulin-like growth factor-1 receptor and augment insulin-like growth factor-1 receptor signaling in cardiac muscle: implications on decreased myocardial protection in diabetic cardiomyopathy. J Biol Chem. 2003; 278:45492–45498. PMID: 12970367.22. Yamashita K, Kajstura J, Discher DJ, Wasserlauf BJ, Bishopric NH, Anversa P, Webster KA. Reperfusion-activated Akt kinase prevents apoptosis in transgenic mouse hearts overexpressing insulin-like growth factor-1. Circ Res. 2001; 88:609–614. PMID: 11282895.
Article23. Russell-Jones DL, Bates AT, Umpleby AM, Hennessy TR, Bowes SB, Hopkins KD, Jackson N, Kelly J, Shojaee-Moradie F, Jones RH, et al. A comparison of the effects of IGF-I and insulin on glucose metabolism, fat metabolism and the cardiovascular system in normal human volunteers. Eur J Clin Invest. 1995; 25:403–411. PMID: 7656918.
Article24. Katz LE, DeLeón DD, Zhao H, Jawad AF. Free and total insulin-like growth factor (IGF)-I levels decline during fasting: relationships with insulin and IGF-binding protein-1. J Clin Endocrinol Metab. 2002; 87:2978–2983. PMID: 12050283.
Article25. Luo J, Murphy LJ. Differential expression of the insulin-like growth factor binding proteins in spontaneously diabetic rats. J Mol Endocrinol. 1992; 8:155–163. PMID: 1381181.
Article26. Lang CH, Vary TC, Frost RA. Acute in vivo elevation of insulin-like growth factor (IGF) binding protein-1 decreases plasma free IGF-I and muscle protein synthesis. Endocrinology. 2003; 144:3922–3933. PMID: 12933666.
Article27. Groban L, Pailes NA, Bennett CD, Carter CS, Chappell MC, Kitzman DW, Sonntag WE. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006; 61:28–35. PMID: 16456192.
Article28. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010; 107:1058–1070. PMID: 21030723.
Article30. Zapf J, Hauri C, Waldvogel M, Froesch ER. Acute metabolic effects and half-lives of intravenously administered insulinlike growth factors I and II in normal and hypophysectomized rats. J Clin Invest. 1986; 77:1768–1775. PMID: 3711334.
Article31. Di Cola G, Cool MH, Accili D. Hypoglycemic effect of insulin-like growth factor-1 in mice lacking insulin receptors. J Clin Invest. 1997; 99:2538–2544. PMID: 9153298.
Article32. Sen P, Mukherjee S, Ray D, Raha S. Involvement of the Akt/PKB signaling pathway with disease processes. Mol Cell Biochem. 2003; 253:241–246. PMID: 14619975.33. Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997; 91:231–241. PMID: 9346240.
Article34. Yu W, Wu J, Cai F, Xiang J, Zha W, Fan D, Guo S, Ming Z, Liu C. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One. 2012; 7:e52013. PMID: 23251674.
Article35. Völkers M, Doroudgar S, Nguyen N, Konstandin MH, Quijada P, Din S, Ornelas L, Thuerauf DJ, Gude N, Friedrich K, Herzig S, Glembotski CC, Sussman MA. PRAS40 prevents development of diabetic cardiomyopathy and improves hepatic insulin sensitivity in obesity. EMBO Mol Med. 2014; 6:57–65. PMID: 24408966.
Article36. Li SY, Fang CX, Aberle NS 2nd, Ren BH, Ceylan-Isik AF, Ren J. Inhibition of PI-3 kinase/Akt/mTOR, but not calcineurin signaling, reverses insulin-like growth factor I-induced protection against glucose toxicity in cardiomyocyte contractile function. J Endocrinol. 2005; 186:491–503. PMID: 16135669.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Resveratrol modulates the Akt/GSK-3β signaling pathway in a middle cerebral artery occlusion animal model
- Anti-diabetic effects of benfotiamine on an animal model of type 2 diabetes mellitus
- IGF-I Exerts an Anti-inflammatory Effect on Skeletal Muscle Cells through Down-regulation of TLR4 Signaling
- Potential involvement of glycogen synthase kinase (GSK)-3β in a rat model of multiple sclerosis: evidenced by lithium treatment
- Curcumin targets vascular endothelial growth factor viaactivating the PI3K/Akt signaling pathway and improves brainhypoxic-ischemic injury in neonatal rats