Clin Exp Otorhinolaryngol.
2016 Sep;9(3):198-205. 10.21053/ceo.2015.01060.
Asian Sand Dust Enhances the Inflammatory Response and Mucin Gene Expression in the Middle Ear
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Hallym University College of Medicine, Seoul, Korea.
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College of Medicine, Seoul, Korea. jjsong23@gmail.com
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Nursing Science, College of Nursing, Gachon University, Incheon, Korea.
- KMID: 2353616
- DOI: http://doi.org/10.21053/ceo.2015.01060
Abstract
OBJECTIVES
Asia sand dust (ASD) is known to cause various human diseases including respiratory infection. The aim of this study was to examine the effect of ASD on inflammatory response in human middle ear epithelial cells (HMEECs) in vitro and in vivo.
METHODS
Cell viability was assessed using the cell counting kit-8 assay. The mRNA levels of various genes including COX-2, TNF-a, MUC 5AC, MUC 5B, TP53, BAX, BCL-2, NOX4, and SOD1 were analyzed using semiquantitative realtime polymerase chain reaction. COX-2 protein levels were determined by western blot analysis. Sprague Dawley rats were used for in vivo investigations of inflammatory reactions in the middle ear epithelium as a result of ASD injection.
RESULTS
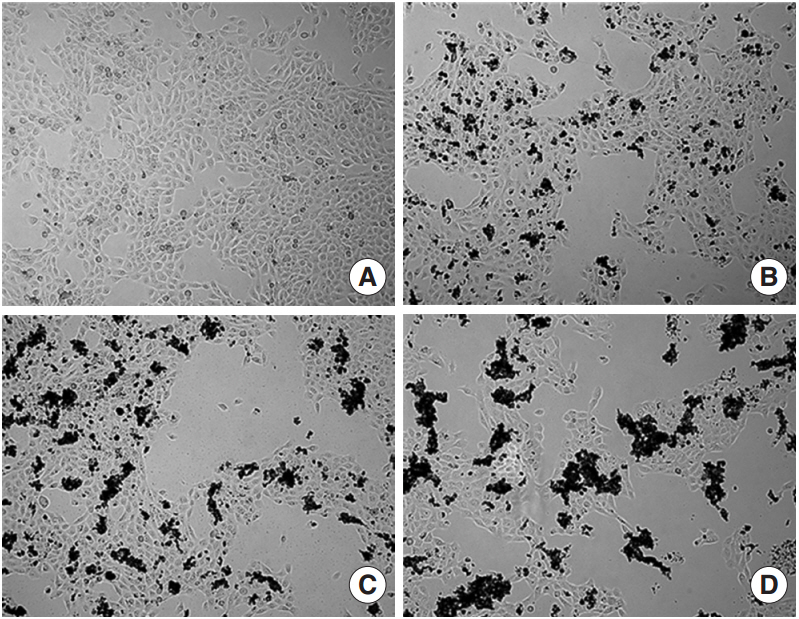
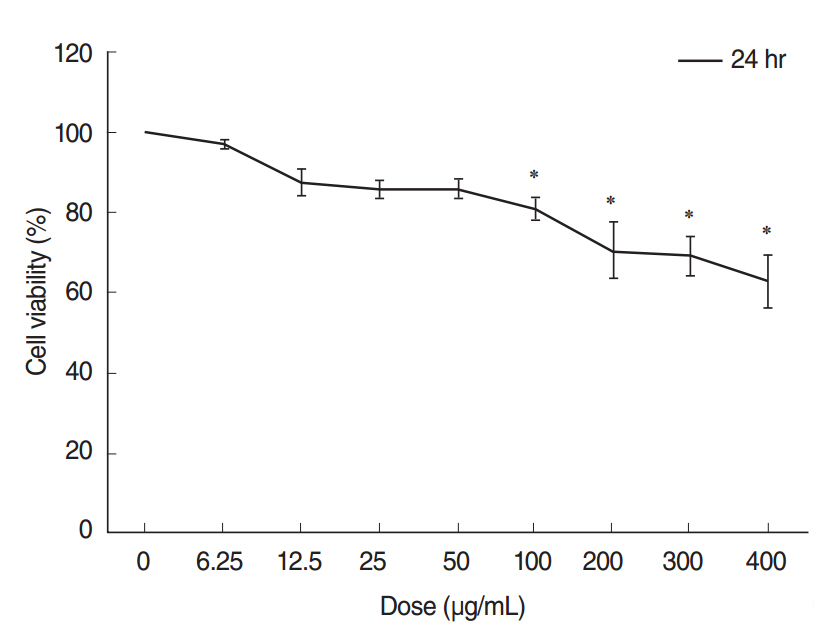
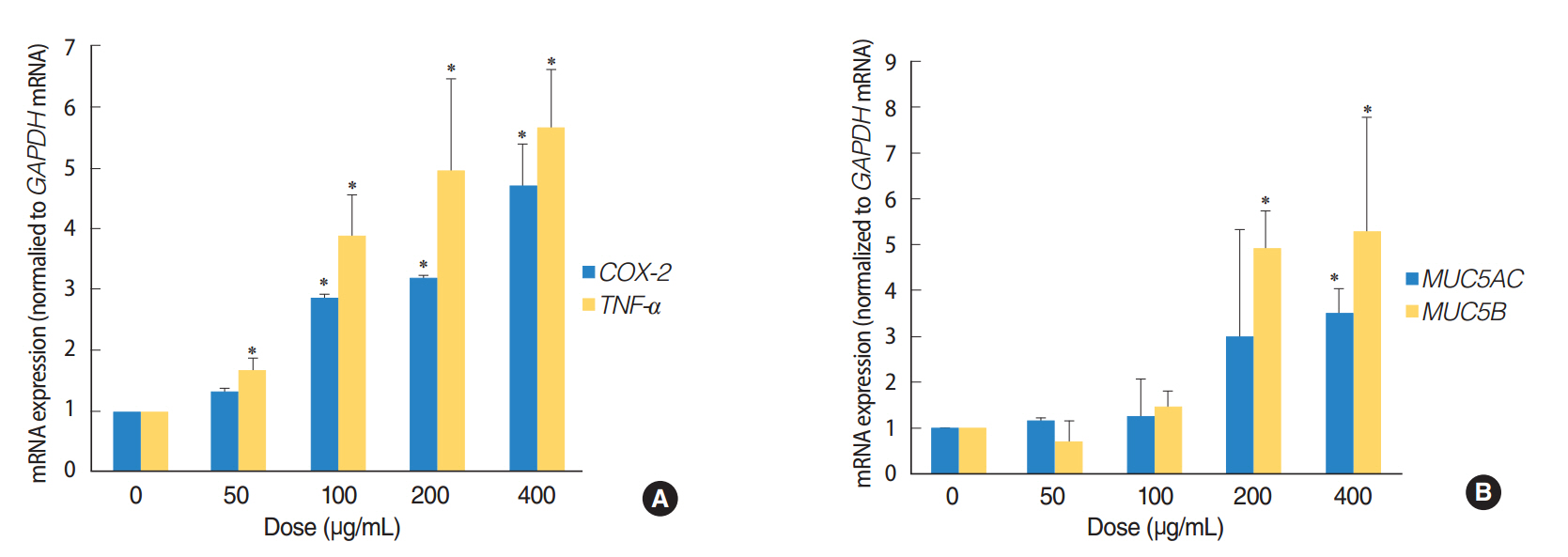
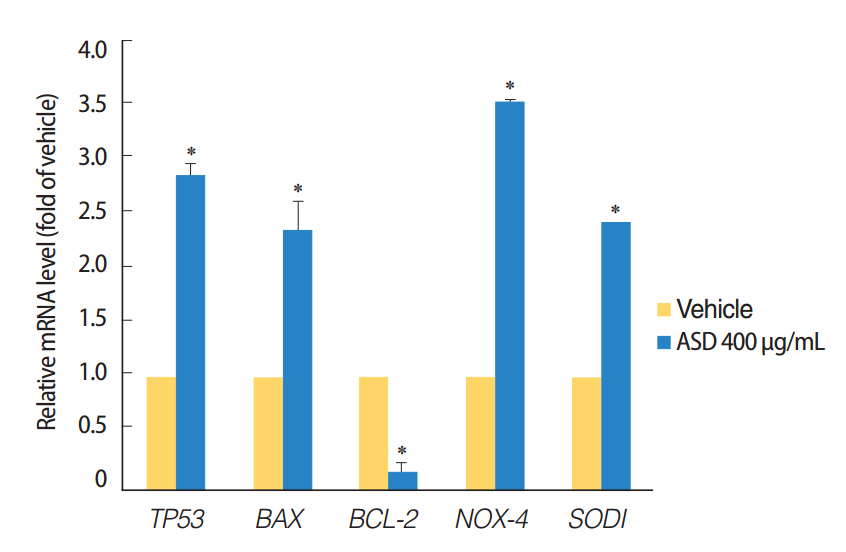
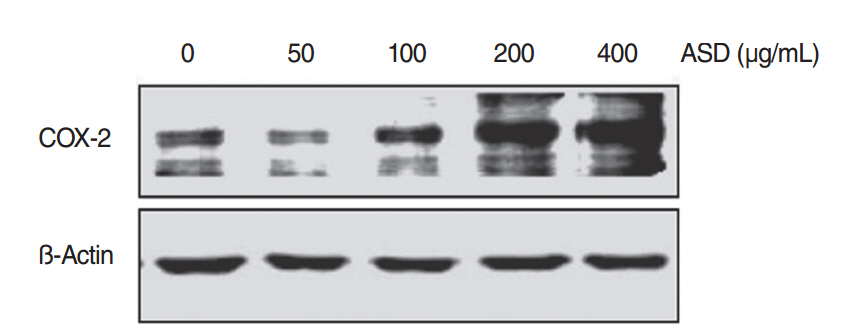
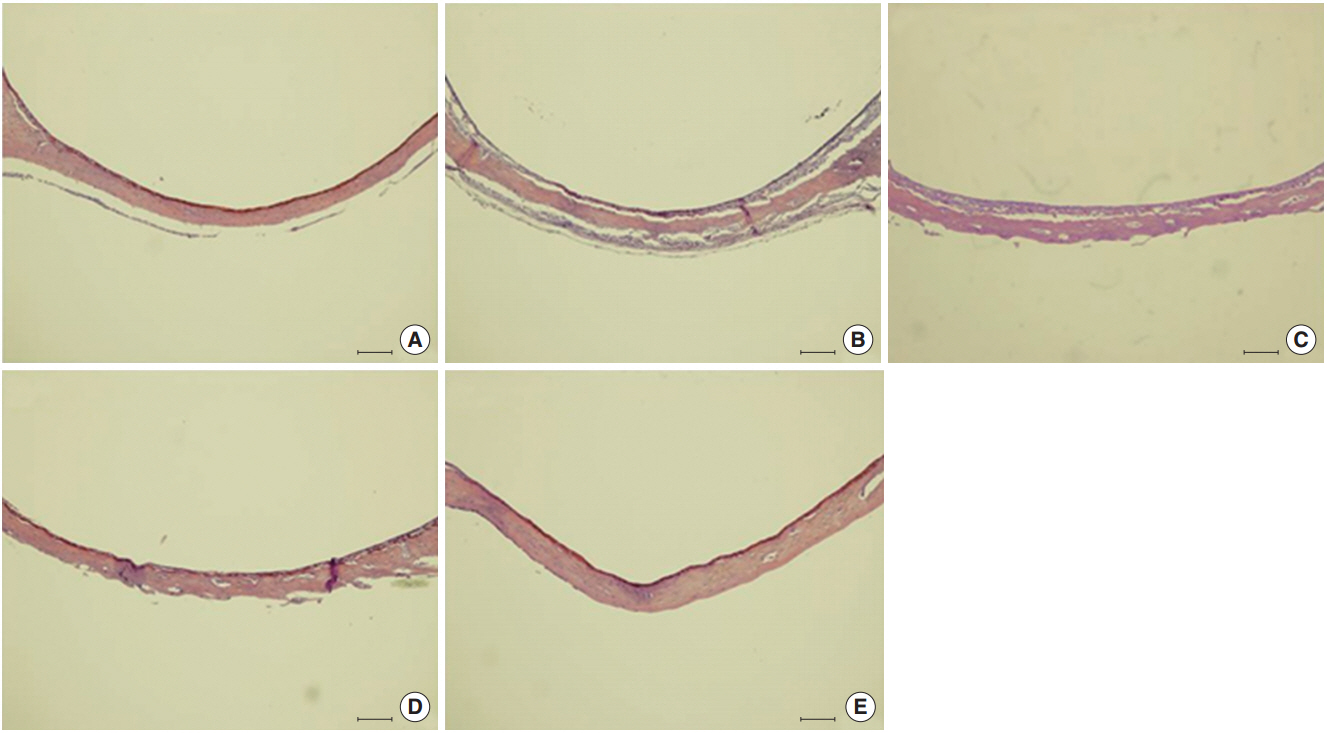
We observed dose-dependent decrease in HMEEC viability. ASD exposure significantly increased COX-2, TNF-a, MUC5AC, and MUC5B mRNA expression. Also, ASD affected the mRNA levels of apoptosis- and oxidative stress-related genes. Western blot analysis revealed a dose-dependent increase in COX-2 production. Animal studies also demonstrated an ASD-induced inflammatory response in the middle ear epithelium.
CONCLUSION
Environmental ASD exposure can result in the development of otitis media.
Keyword
MeSH Terms
-
Animals
Asia
Asian Continental Ancestry Group*
Blotting, Western
Cell Count
Cell Survival
Cyclooxygenase 2
Dust*
Ear, Middle*
Epithelial Cells
Epithelium
Gene Expression*
Humans
In Vitro Techniques
Mucins*
Otitis Media
Polymerase Chain Reaction
Rats, Sprague-Dawley
RNA, Messenger
Cyclooxygenase 2
Dust
Mucins
RNA, Messenger
Figure
Reference
-
1. Yadav MK, Chae SW, Song JJ. In Vitro Streptococcus pneumoniae biofilm formation and in vivo middle ear mucosal biofilm in a rat model of acute otitis induced by S. pneumoniae. Clin Exp Otorhinolaryngol. 2012; Sep. 5(3):139–44.2. Ribeiro H, Cardoso MR. Air pollution and children’s health in São Paulo (1986-1998). Soc Sci Med. 2003; Dec. 57(11):2013–22.
Article3. Dostal M, Hertz-Picciotto I, James R, Keller J, Dejmek J, Selevan S, et al. Childhood morbidity and air pollution in the Teplice program. Cas Lek Cesk. 2001; Oct. 140(21):658–61.4. Rovers MM, de Kok IM, Schilder AG. Risk factors for otitis media: an international perspective. Int J Pediatr Otorhinolaryngol. 2006; Jul. 70(7):1251–6.
Article5. Heinrich J, Frye C, Holscher B, Meyer I, Pitz M, Cyrys J, et al. Environmental surveys in the areas of Bitterfeld, Hettstedt and a comparative area in 1992-2000. Gesundheitswesen. 2002; Dec. 64(12):675–82.6. Kang IG, Jung JH, Kim ST. Asian sand dust enhances allergen-induced th2 allergic inflammatory changes and mucin production in BALB/c mouse lungs. Allergy Asthma Immunol Res. 2012; Jul. 4(4):206–13.
Article7. Chen YS, Yang CY. Effects of Asian dust storm events on daily hospital admissions for cardiovascular disease in Taipei, Taiwan. J Toxicol Environ Health A. 2005; Sep. 68(17-18):1457–64.
Article8. Honda A, Matsuda Y, Murayama R, Tsuji K, Nishikawa M, Koike E, et al. Effects of Asian sand dust particles on the respiratory and immune system. J Appl Toxicol. 2014; Mar. 34(3):250–7.
Article9. Chun YM, Moon SK, Lee HY, Webster P, Brackmann DE, Rhim JS, et al. Immortalization of normal adult human middle ear epithelial cells using a retrovirus containing the E6/E7 genes of human papillomavirus type 16. Ann Otol Rhinol Laryngol. 2002; Jun. 111(6):507–17.
Article10. Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health. 2013; May. 12(1):43.
Article11. Huang YC, Karoly ED, Dailey LA, Schmitt MT, Silbajoris R, Graff DW, et al. Comparison of gene expression profiles induced by coarse, fine, and ultrafine particulate matter. J Toxicol Environ Health A. 2011; 74(5):296–312.
Article12. Hiyoshi K, Ichinose T, Sadakane K, Takano H, Nishikawa M, Mori I, et al. Asian sand dust enhances ovalbumin-induced eosinophil recruitment in the alveoli and airway of mice. Environ Res. 2005; Nov. 99(3):361–8.
Article13. Caceres Udina MJ, Alvarez Martinez JA, Argente del Castillo J, Chumilla Valderas MA, Fernandez Alvarez E, Garrido Romera A, et al. Incidence, air pollution and risk factors of acute otitis media in the first year of life: a prospective study. An Pediatr (Barc). 2004; Feb. 60(2):133–8.14. Preciado D, Kuo E, Ashktorab S, Manes P, Rose M. Cigarette smoke activates NFκB-mediated Tnf-α release from mouse middle ear cells. Laryngoscope. 2010; Dec. 120(12):2508–15.
Article15. Kakiuchi M, Tsujigiwa H, Orita Y, Nagatsuka H, Yoshinobu J, Kariya S, et al. Cyclooxygenase 2 expression in otitis media with effusion. Am J Otolaryngol. 2006; Mar-Apr. 27(2):81–5.
Article16. Medeiros R, Figueiredo CP, Pandolfo P, Duarte FS, Prediger RD, Passos GF, et al. The role of TNF-alpha signaling pathway on COX-2 upregulation and cognitive decline induced by beta-amyloid peptide. Behav Brain Res. 2010; May. 209(1):165–73.17. Kim ST, Ye MK, Shin SH. Effects of Asian sand dust on mucin gene expression and activation of nasal polyp epithelial cells. Am J Rhinol Allergy. 2011; Sep-Oct. 25(5):303–6.
Article18. Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010; Dec. 363(23):2233–47.
Article19. Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014; Jan. 505(7483):412–6.
Article20. Kawano H, Paparella MM, Ho SB, Schachern PA, Morizono N, Le CT, et al. Identification of MUC5B mucin gene in human middle ear with chronic otitis media. Laryngoscope. 2000; Apr. 110(4):668–73.
Article21. Cho JG, Woo JS, Lee HM, Jung HH, Hwang SJ, Chae S. Effects of cigarette smoking on mucin production in human middle ear epithelial cells. Int J Pediatr Otorhinolaryngol. 2009; Oct. 73(10):1447–51.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microarray Analysis of Gene Expression Alteration in Human Middle Ear Epithelial Cells Induced by Asian Sand Dust
- Asian Sand Dust Enhances Allergen-Induced Th2 Allergic Inflammatory Changes and Mucin Production in BALB/c Mouse Lungs
- From Historical Dust ail to Early Warning of Asian Dust Events in Korea
- The Health Effects of Asian Dust Event
- Physical and Chemical Characteristics of Asian Dust