Transl Clin Pharmacol.
2015 Dec;23(2):66-74. 10.12793/tcp.2015.23.2.66.
Population pharmacokinetic analysis of the multiple peaks phenomenon in sumatriptan
- Affiliations
-
- 1Clinical Trial Center, Department of Biomedical Science and BK21 Plus Program, Kyungpook National University Hospital and School, Daegu 41944, Republic of Korea.
- 2College of Pharmacy, Yeungnam University, Gyeongsan 42415, Korea.
- 3PIPET (Pharmacometrics Institute for Practical Education and Training), The Catholic University of Korea, Seoul 06591, Republic of Korea.
- 4Department of Pharmacology, Kosin University College of Medicine, Busan 49267, Korea.
- 5Department of Biostatistics and Bioinformatics, Pharma Partnering Inc., Seoul 06735, Republic of Korea. yooheedoo@gmail.com
- KMID: 2351811
- DOI: http://doi.org/10.12793/tcp.2015.23.2.66
Abstract
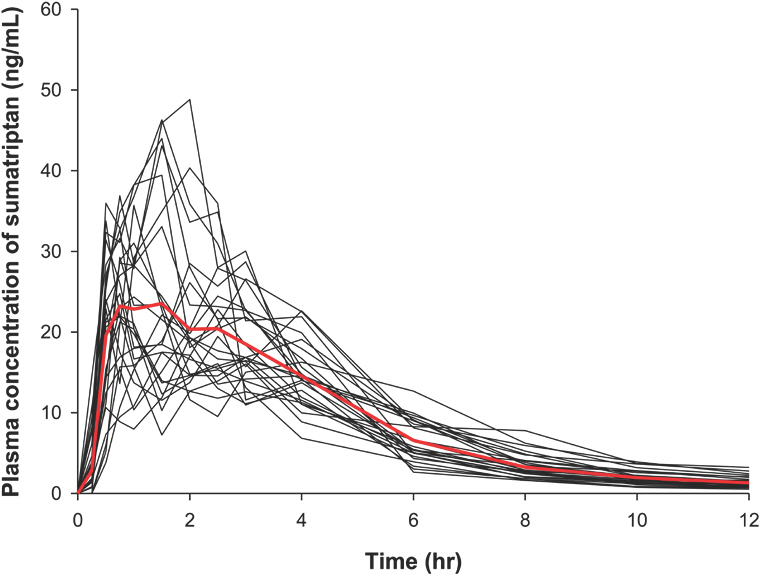
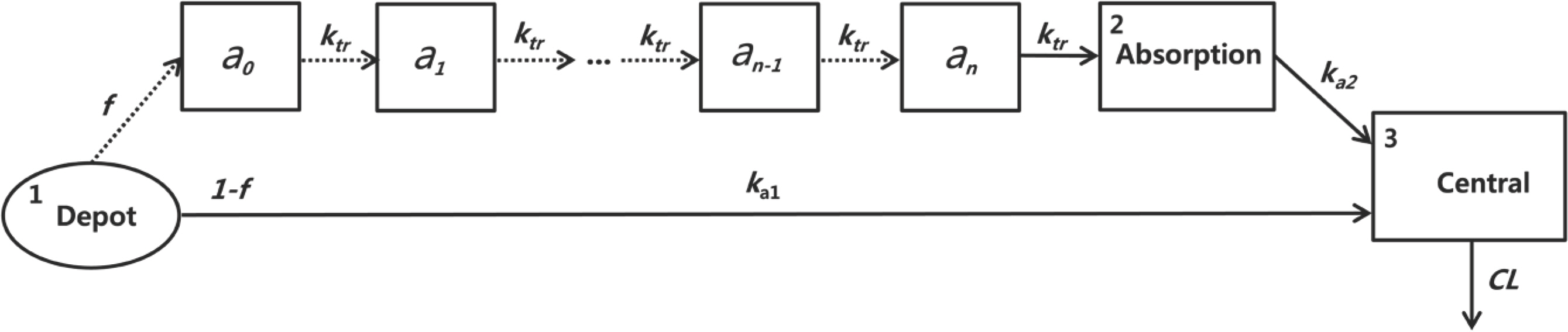
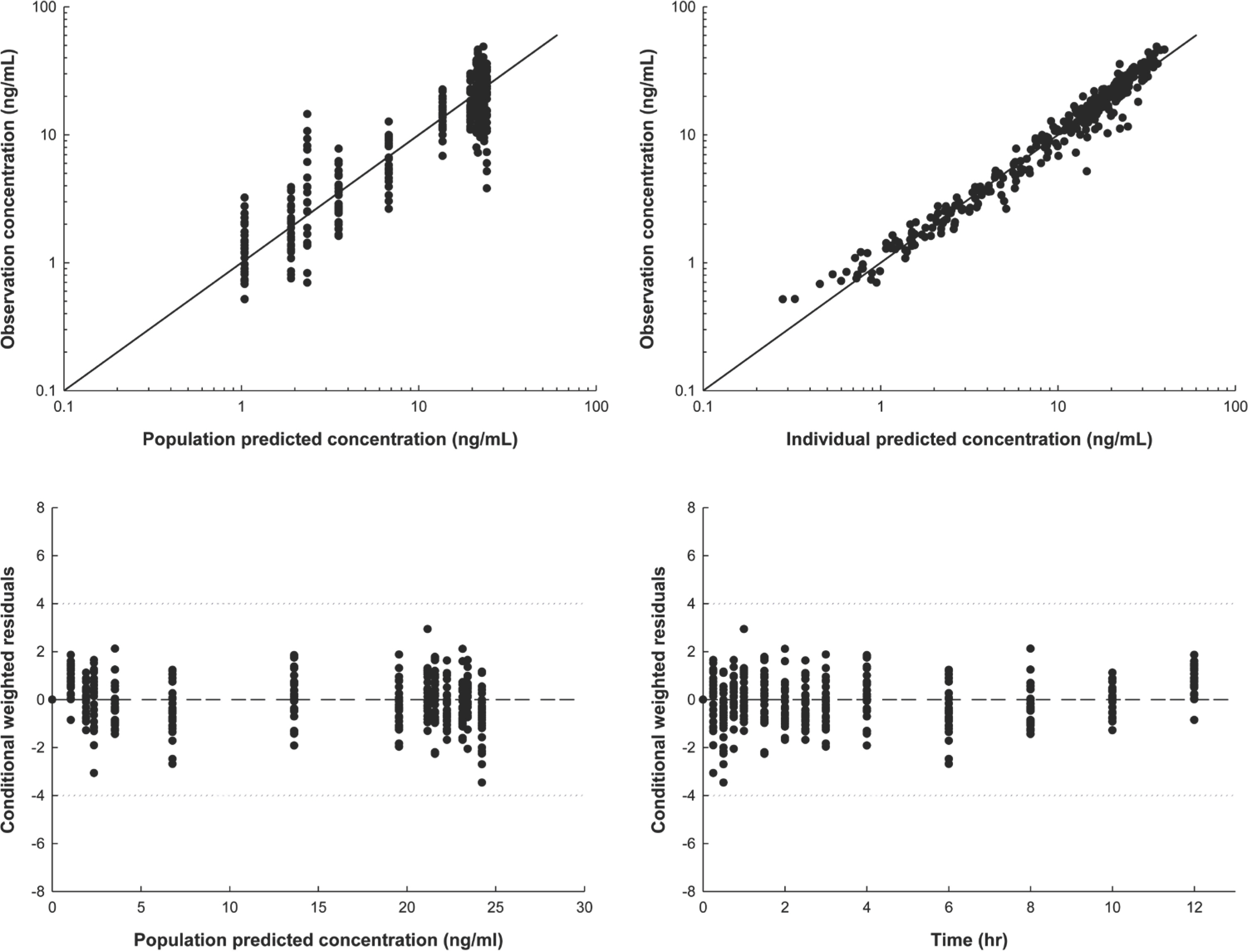
- The objective of this study was to develop a population pharmacokinetic (PK) model for sumatriptan, which frequently shows an atypical absorption profile with multiple peaks. Sumatriptan, a selective agonist for the vascular serotonin (5-HT1) receptor that causes vasoconstriction of the cerebral arteries, is used for the acute treatment of migraine attack with or without aura. Despite its relatively high between-subject variability, few reports have addressed PK modeling of sumatriptan. Plasma data obtained after a single 50-mg oral dose of sumatriptan in 26 healthy Korean male subjects were used. Blood samples were collected 0 (predose), 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, and 12 h after dosing. Plasma sumatriptan concentrations were analyzed using UPLC/MS/MS. Population PK analysis was performed using plasma concentration data for sumatriptan with NONMEM (ver. 7.2). A total of 364 concentrations of sumatriptan were captured by a one-compartment model with first-order elimination, and a combined transit compartment model and first-order absorption with lag time was successful in describing the PK with multiple peaks in the absorption phase of sumatriptan. The creatinine clearance as a covariate significantly (P < 0.01) influenced the absorption fraction (f ). The final model was validated through a visual predictive check and bootstrapping with no serious model misspecification.
MeSH Terms
Figure
Cited by 1 articles
-
Determination of sumatriptan in human plasma using liquid chromatography-mass spectrometry for pharmacokinetic study in healthy Korean volunteers
Seungil Cho, Moonyoung Jegal, Boram Ohk, Bo Kyung Kim, Mi-Ri Gwon, Woo Youl Kang, Sook Jin Seong, Hyun-Ju Kim, Hae Won Lee, Young-Ran Yoon
Transl Clin Pharmacol. 2017;25(2):106-111. doi: 10.12793/tcp.2017.25.2.106.
Reference
-
References
1. Ferrari A, Pinetti D, Bertolini A, Coccia C, Sternieri E. Interindividual variability of oral sumatriptan pharmacokinetics and of clinical response in migraine patients. Eur J Clin Pharmacol. 2008; 64:489–495. doi: 10.1007/s00228-007-0443-9.
Article2. Sternieri E, Pinetti D, Coccia CP, Leone S, Bertolini A, Ferrari A. Pharmacokinetics of sumatriptan in non-respondent and in adverse drug reaction reporting migraine patients. J Headache Pain. 2005; 6:319–321.
Article3. Moore KH, Leese PT, McNeal S, Gray P, O'Quinn S, Bye C, et al. The pharmacokinetics of sumatriptan when administered with clarithromycin in healthy volunteers. Clin Ther. 2002; 24:583–594.
Article4. Rani PU, Naidu MUR, Kumar TR, Shobha JC, Vijay T, Sekhar KR, et al. A bioequivalence study of two brands of sumatriptan tablets. Curr Ther Res Clin E. 1996; 57:589–598.5. Warner PE, Brouwer KL, Hussey EK, Dukes GE, Heizer WD, Donn KH, et al. Sumatriptan absorption from different regions of the human gastrointes-tinaltract. Pharm Res. 1995; 12:138–143.6. Cosson VF, Fuseau E. Mixed effect modeling of sumatriptan pharmacokinetics during drug development: II. From healthy subjects to phase 2 dose ranging in patients. J Pharmacokinet Biopharm. 1999; 27:149–171.7. Seo JJ, Park J, Bae MH, Lim MS, Seong SJ, Lee J, et al. Rapid determination of sumatriptan in human plasma by ultra performance liquid chromatography-tandem mass spectrometry and its application to clinical pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2013; 919-920:38–42. doi: 10.1016/j.jchromb.2013.01.004.
Article8. Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007; 34:711–726.
Article9. van der Walt JS, Hong Y, Zhang L, Pfister M, Boulton DW, Karlsson MO. A Nonlinear Mixed Effects Pharmacokinetic Model for Dapagliflozin and Dapagliflozin 3-O-glucuronide in Renal or Hepatic Impairment. CPT Pharmacometrics Syst Pharmacol. 2013; 2:e42. doi: 10.1038/psp.2013.20.
Article10. Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Biopharm. 1994; 22:431–445.
Article12. Stefano FJ, Trendelenburg U. Saturation of monoamine oxidase by intra-neuronal noradrenaline accumulation. Naunyn Schmiedebergs Arch Pharmacol. 1984; 328:135–141.
Article13. Donnelly CH, Murphy DL. Substrate-and inhibitor-related characteristics of human platelet monoamine oxidase. Biochem Pharmacol. 1977; 26:853–858.14. Davies NM, Takemoto JK, Brocks DR, Yáñez JA. Multiple peaking phenomena in pharmacokinetic disposition. Clin Pharmacokinet. 2010; 49:351–377. doi: 10.2165/11319320-000000000-00000.
Article15. Aurora S, Kori S, Barrodale P, Nelsen A, McDonald S. Gastric stasis occurs in spontaneous, visually induced, and interictal migraine. Headache. 2007; 47:1443–1446.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Determination of sumatriptan in human plasma using liquid chromatography-mass spectrometry for pharmacokinetic study in healthy Korean volunteers
- PKconverter: R package to convert the pharmacokinetic parameters
- Oral Sumatriptan for Acute Treatment of Migraine A Single-blind Placebo-controlled Study
- A Case of Vasospastic Angina Pectoris Occurring Following Administration of Oral Imigran(R) for Migraine
- A Case of Cyclic Vomiting Syndrome Improved by Sumatriptan