Immune Netw.
2016 Aug;16(4):201-210. 10.4110/in.2016.16.4.201.
STAT6 and PARP Family Members in the Development of T Cell-dependent Allergic Inflammation
- Affiliations
-
- 1Department of Pediatrics, Wells Center for Pediatric Research, Indianapolis, IN 46202, USA. mkaplan2@iu.edu
- 2Department of Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
- KMID: 2349881
- DOI: http://doi.org/10.4110/in.2016.16.4.201
Abstract
- Allergic inflammation requires the orchestration of altered gene expression in the target tissue and in the infiltrating immune cells. The transcription factor STAT6 is critical in activating cytokine gene expression and cytokine signaling both in the immune cells and in target tissue cells including airway epithelia, keratinocytes and esophageal epithelial cells. STAT6 is activated by the cytokines IL-4 and IL-13 to mediate the pathogenesis of allergic disorders such as asthma, atopic dermatitis, food allergy and eosinophilic esophagitis (EoE). In this review, we summarize the role of STAT6 in allergic diseases, its interaction with the co-factor PARP14 and the molecular mechanisms by which STAT6 and PARP14 regulate gene transcription.
Keyword
MeSH Terms
Figure
Reference
-
1. Levy DE, Darnell JE. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002; 3:651–662.
Article2. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008; 454:445–454.
Article3. Quelle FW, Shimoda K, Thierfelder W, Fischer C, Kim A, Ruben SM, Cleveland JL, Pierce JH, Keegan AD, Nelms K, Paul WE, Ihle JN. Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL-4 and IL-3 but are not required for mitogenesis. Mol Cell Biol. 1995; 15:3336–3343.
Article4. Mikita T, Campbell D, Wu P, Williamson K, Schindler U. Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol Cell Biol. 1996; 16:5811–5820.
Article5. Wurster AL, Tanaka T, Grusby MJ. The biology of Stat4 and Stat6. Oncogene. 2000; 19:2577–2584.
Article6. Hebenstreit D, Wirnsberger G, Horejs-hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006; 17:173–188.7. Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996; 380:627–630.8. Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, Doherty PC, Grosveld G, Paul WE, Ihle JN. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996; 380:630–633.
Article9. Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for the development of Th2 cells. Immunity. 1996; 4:313–319.
Article10. Ouyang W, Löhning M, Gao Z, Assenmacher M, Ranganath S, Radbruch a, Murphy KM. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000; 12:27–37.
Article11. Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998; 187:939–948.
Article12. Akimoto T, Numata F, Tamura M, Takata Y, Higashida N, Takashi T, Takeda K, Akira S. Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6-deficient mice. J Exp Med. 1998; 187:1537–1542.
Article13. Tomkinson A, Kanehiro A, Rabinovitch N, Joetham A, Cieslewicz G, Gelfand EW. The failure of STAT6-deficient mice to develop airway eosinophilia and airway hyperresponsiveness is overcome by interleukin-5. Am J Respir Crit Care Med. 1999; 160:1283–1291.
Article14. Webb DC, McKenzie AN, Koskinen AM, Yang M, Mattes J, Foster PS. Integrated signals between IL-13, IL-4, and IL-5 regulate airways hyperreactivity. J Immunol. 2000; 165:108–113.
Article15. Hoshino A, Tsuji T, Matsuzaki J, Jinushi T, Ashino S, Teramura T, Chamoto K, Tanaka Y, Asakura Y, Sakurai T, Mita Y, Takaoka A, Nakaike S, Takeshima T, Ikeda H, Nishimura T. STAT6-mediated signaling in Th2-dependent allergic asthma: Critical role for the development of eosinophilia, airway hyper-responsiveness and mucus hypersecretion, distinct from its role in Th2 differentiation. Int Immunol. 2004; 16:1497–1505.
Article16. Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999; 104:1001–1006.
Article17. Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002; 8:885–889.
Article18. McCusker CT, Wang Y, Shan J, Kinyanjui MW, Villeneuve A, Michael H, Fixman ED. Inhibition of experimental allergic airways disease by local application of a cell-penetrating dominant-negative STAT-6 peptide. J Immunol. 2007; 179:2556–2564.
Article19. Chiba Y, Todoroki M, Nishida Y, Tanabe M, Misawa M. A novel STAT6 inhibitor AS1517499 ameliorates antigen-induced bronchial hypercontractility in mice. Am J Respir Cell Mol Biol. 2009; 41:516–524.
Article20. Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994; 94:870–876.
Article21. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O'Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, McLean WH. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006; 38:441–446.22. Baurecht H, Irvine AD, Novak N, Illig T, Bühler B, Ring J, Wagenpfeil S, Weidinger S. Toward a major risk factor for atopic eczema: meta-analysis of filaggrin polymorphism data. J Allergy Clin Immunol. 2007; 120:1406–1412.
Article23. Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, Schneider L, Beck LA, Barnes KC, Leung DY. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007; 120:150–155.24. Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008; 126:332–337.
Article25. Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, Darst MA, Gao B, Boguniewicz M, Travers JB, Leung DY. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003; 171:3262–3269.
Article26. Bruns HA, Schindler U, Kaplan MH. Expression of a constitutively active Stat6 in vivo alters lymphocyte homeostasis with distinct effects in T and B cells. J Immunol. 2003; 170:3478–3487.
Article27. Sehra S, Bruns HA, Ahyi AN, Nguyen ET, Schmidt NW, Michels EG, von Bülow GU, Kaplan MH. IL-4 is a critical determinant in the generation of allergic inflammation initiated by a constitutively active Stat6. J Immunol. 2008; 180:3551–3559.
Article28. Sehra S, Yao Y, Howell MD, Nguyen ET, Kansas GS, Leung DY, Travers JB, Kaplan MH. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010; 184:3186–3190.
Article29. Turner MJ, Dasilva-arnold S, Luo N, Hu X, West CC, Sun L, Hall C, Bradish J, Kaplan MH, Travers JB, Sun Y. STAT6-mediated keratitis and blepharitis : a novel murine model of ocular atopic dermatitis. Invest Ophthalmol Vis Sci. 2014; 55:3803–3808.
Article30. Chan LS, Robinson N, Xu L. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J Invest Dermatol. 2001; 117:977–983.
Article31. Zheng T, Oh MH, Oh SY, Schroeder JT, Glick AB, Zhu Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J Invest Dermatol. 2009; 129:742–751.
Article32. Sicherer SH, Leung DY. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2014. J Allergy Clin Immunol. 2015; 135:357–367.
Article33. Amoli MM, Hand S, Hajeer AH, Jones KP, Rolf S, Sting C, Davies BH, Ollier WE. Polymorphism in the Stat6 gene encodes risk for nut allergy. Genes Immun. 2002; 3:220–224.
Article34. Hancock DB, Romieu I, Chiu GY, Sienra-Monge JJ, Li H, Estela Del Rio-Navarro B, London SJ. STAT6 and LRP1 polymorphisms are associated with food allergen sensitization in Mexican children. J Allergy Clin Immunol. 2012; 129:1673–1676.
Article35. Kweon MN, Yamamoto M, Kajiki M, Takahashi I, Kiyono H. Systemically derived large intestinal CD4 + Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest. 2000; 106:199–206.
Article36. Brandt EB, Munitz A, Orekov T, Mingler MK, Mcbride M, Finkelman FD, Rothenberg ME. Targeting IL-4 / IL-13 signaling to alleviate oral allergen - induced diarrhea. J Allergy Clin Immunol. 2009; 123:53–58.37. Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, Chatila TA, Oettgen HC. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol. 2013; 6:740–750.
Article38. Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, Ahrens R, Seidu L, Mckenzie A, Strait R, Finkelman FD, Foster PS, Matthaei KI, Rothenberg ME, Hogan SP. IL-9 - and mast cell - mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008; 205:897–913.
Article39. Chen CY, Lee JB, Liu B, Ohta S, Wang PY, Kartashov AV, Mugge L, Abonia JP, Barski A, Izuhara K, Rothenberg ME, Finkelman FD, Hogan SP. Induction of Interleukin-9-Producing Mucosal Mast Cells Promotes Susceptibility to IgE-Mediated Experimental Food Allergy. Immunity. 2015; 43:788–802.
Article40. Mathias CB, Hobson SA, Garcia-lloret M, Lawson G, Poddighe D, Freyschmidt EJ, Xing W, Gurish MF, Chatila TA, Oettgen HC. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J Allergy Clin Immunol. 2011; 127:795–805.e1-6.
Article41. Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, Rachid R, Chatila TA. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015; 42:512–523.
Article42. Blanchard C, Durual S, Estienne M, Emami S, Vasseur S, Cuber JC. Eotaxin-3/CCL26 gene expression in intestinal epithelial cells is up-regulated by interleukin-4 and interleukin-13 via the signal transducer and activator of transcription 6. Int J Biochem Cell Biol. 2005; 37:2559–2573.
Article43. Furuta GT, Katzka DA. Eosinophilic Esophagitis. N Engl J Med. 2015; 373:1640–1648.
Article44. Hoeck J, Woisetschläger M. Activation of eotaxin-3/CCLl26 gene expression in human dermal fibroblasts is mediated by STAT6. J Immunol. 2001; 167:3216–3222.
Article45. Kagami S, Saeki H, Komine M, Kakinuma T, Tsunemi Y, Nakamura K, Sasaki K, Asahina A, Tamaki K. Interleukin-4 and interleukin-13 enhance CCL26 production in a human keratinocyte cell line, HaCaT cells. Clin Exp Immunol. 2005; 141:459–466.
Article46. Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003; 125:1419–1427.
Article47. Zhang X, Cheng E, Huo X, Yu C, Zhang Q, Pham TH, Wang DH, Spechler SJ, Souza RF. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS One. 2012; 7:e50037.
Article48. Niranjan R, Rayapudi M, Mishra A, Dutt P, Dynda S, Mishra A. Pathogenesis of allergen-induced eosinophilic esophagitis is independent of interleukin (IL)-13. Immunol Cell Biol. 2013; 91:408–415.
Article49. Krishnamurthy P, Sherrill JD, Parashette K, Goenka S, Rothenberg ME, Gupta S, Kaplan MH. Correlation of increased PARP14 and CCL26 expression in biopsies from children with eosinophilic esophagitis. J Allergy Clin Immunol. 2014; 133:577–580.
Article50. Blanchard C, Stucke EM, Burwinkel K, Caldwell JM, Collins MH, Ahrens A, Buckmeier BK, Jameson SC, Greenberg A, Kaul A, Franciosi JP, Kushner JP, Martin LJ, Putnam PE, Abonia JP, Wells SI, Rothenberg ME. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol. 2010; 184:4033–4041.
Article51. Sherrill JD, Kc K, Wu D, Djukic Z, Caldwell JM, Stucke EM, Kemme KA, Costello MS, Mingler MK, Blanchard C, Collins MH, Abonia JP, Putnam PE, Dellon ES, Orlando RC, Hogan SP, Rothenberg ME. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2014; 7:718–729.
Article52. Hottiger MO, Hassa PO, Lüscher B, Schüler H, Koch-Nolte F. Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends Biochem Sci. 2010; 35:208–219.53. Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006; 7:517–528.
Article54. Yélamos J, Monreal Y, Saenz L, Aguado E, Schreiber V, Mota R, Fuente T, Minguela A, Parrilla P, de Murcia G, Almarza E, Aparicio P, Ménissier-de Murcia J. PARP-2 deficiency affects the survival of CD4+CD8+ double-positive thymocytes. EMBO J. 2006; 25:4350–4360.
Article55. Rosado MM, Bennici E, Novelli F, Pioli C. Beyond DNA repair, the immunological role of PARP-1 and its siblings. Immunology. 2013; 139:428–437.
Article56. Saenz L, Lozano JJ, Valdor R, Baroja-Mazo A, Ramirez P, Parrilla P, Aparicio P, Sumoy L, Yélamos J. Transcriptional regulation by poly (ADP-ribose) polymerase-1 during T cell activation. BMC Genomics. 2008; 9:171.57. Kim MY, Zhang T, Kraus WL. Poly (ADP-ribosyl) ation by PARP-1 : "PAR-laying" NAD + into a nuclear signal. Genes Dev. 2005; 19:1951–1967.58. Oumouna M, Datta R, Oumouna-Benachour K, Suzuki Y, Hans C, Matthews K, Fallon K, Boulares H. Poly(ADP-ribose) polymerase-1 inhibition prevents eosinophil recruitment by modulating Th2 cytokines in a murine model of allergic airway inflammation: a potential specific effect on IL-5. J Immunol. 2006; 177:6489–6496.
Article59. Boulares AH, Zoltoski AJ, Sherif ZA, Jolly P, Massaro D, Smulson ME. Gene knockout or pharmacological inhibition of poly(ADP-ribose) polymerase-1 prevents lung inflammation in a murine model of asthma. Am J Respir Cell Mol Biol. 2003; 28:322–329.
Article60. Suzuki Y, Masini E, Mazzocca C, Cuzzocrea S, Ciampa A, Suzuki H, Bani D. Inhibition of Poly (ADP-Ribose) polymerase prevents allergen- induced asthma-like reaction in sensitized guinea pigs. J Pharmacol Exp Ther. 2004; 311:1241–1248.
Article61. Virág L, Bai P, Bak I, Pacher P, Mabley JG, Liaudet L, Bakondi E, Gergely P, Kollai M, Szabó C. Effects of poly(ADP-ribose) polymerase inhibition on inflammatory cell migration in a murine model of asthma. Med Sci Monit. 2004; 10:BR77–BR83.62. Naura AS, Hans CP, Zerfaoui M, You D, Cormier SA, Oumouna M, Boulares AH. Post-allergen challenge inhibition of poly(ADP-ribose) polymerase harbors therapeutic potential for treatment of allergic airway inflammation. Clin Exp Allergy. 2008; 38:839–846.
Article63. Olabisi OA, Soto-Nieves N, Nieves E, Yang TT, Yang X, Yu RY, Suk HY, Macian F, Chow CW. Regulation of transcription factor NFAT by ADP-ribosylation. Mol Cell Biol. 2008; 28:2860–2871.
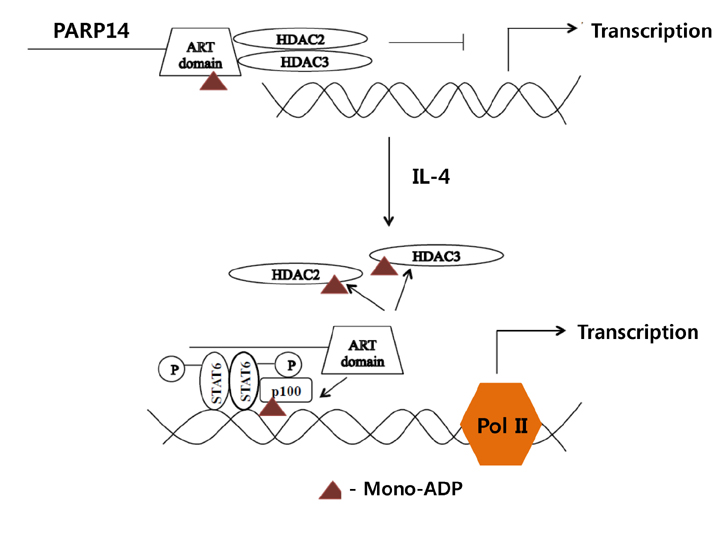
Article64. Datta R, Naura AS, Zerfaoui M, Errami Y, Oumouna M, Kim H, Ju J, Ronchi VP, Haas AL, Boulares AH. PARP-1 deficiency blocks IL-5 expression through calpain-dependent degradation of STAT-6 in a murine asthma model. Allergy. 2011; 66:853–861.
Article65. Pehrson JR, Fried VA. MacroH2A, a core histone containing a large nonhistone region. Science. 1992; 5075:1398–1400.
Article66. Ladurner AG. Inactivating chromosomes: A macro domain that minimizes transcription. Mol Cell. 2003; 12:1–3.67. Vyas S, Matic I, Uchima L, Rood J, Zaja R, Hay RT, Ahel I, Chang P. Family-wide analysis of poly(ADP-ribose) polymerase activity. Nat Commun. 2014; 5:4426.
Article68. Goenka S, Boothby M. Selective potentiation of Stat-dependent gene expression by collaborator of Stat6 (CoaSt6), a transcriptional cofactor. Proc Natl Acad Sci U S A. 2006; 103:4210–4215.
Article69. Goenka S, Cho SH, Boothby M. Collaborator of Stat6 (CoaSt6)-associated poly(ADP-ribose) polymerase activity modulates Stat6-dependent gene transcription. J Biol Chem. 2007; 282:18732–18739.
Article70. Mehrotra P, Riley JP, Patel R, Li F, Voss L, Goenka S. PARP-14 functions as a transcriptional switch for Stat6-dependent gene activation. J Biol Chem. 2011; 286:1767–1776.
Article71. Mehrotra P, Hollenbeck A, Riley JP, Li F, Patel RJ, Akhtar N, Goenka S. Poly (ADP-ribose) polymerase 14 and its enzyme activity regulates TH2 differentiation and allergic airway disease. J Allergy Clin Immunol. 2013; 131:521–531.
Article72. Bettelli E, Korn T, Kuchroo VK. Th17 : the third member of the effector T cell trilogy. Curr Opin Immunol. 2007; 19:652–657.73. Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010; 72:495–516.74. Kemeny DM. The role of the T follicular helper cells in allergic disease. Cell Mol Immunol. 2012; 9:386–389.
Article75. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014; 41:529–542.
Article76. Riley JP, Kulkarni A, Mehrotra P, Koh B, Perumal NB, Kaplan MH, Goenka S. PARP-14 binds specific DNA sequences to promote Th2 cell gene expression. PLoS One. 2013; 8:e83127.
Article77. Mehrotra P, Krishnamurthy P, Sun J, Goenka S, Kaplan MH. Poly-ADP-ribosyl polymerase-14 promotes T helper 17 and follicular T helper development. Immunology. 2015; 146:537–546.
Article78. Cho SH, Goenka S, Henttinen T, Gudapati P, Reinikainen A, Eischen CM, Lahesmaa R, Boothby M. PARP-14, a member of the B aggressive lymphoma family, transduces survival signals in primary B cells. Blood. 2009; 113:2416–2425.
Article79. Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa'ad AH, Putnam PE, Aronow BJ, Rothenberg ME. Eotaxin-3 and a uniquely conserved gene expression profile in eosinophilic esophagitis. J Clin Invest. 2006; 116:536–547.
Article80. Gupta SK, Fitzgerald JF, Kondratyuk T, HogenEsch H. Cytokine expression in normal and inflamed esophageal mucosa: a study into the pathogenesis of allergic eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2006; 42:22–26.
Article81. Nasta F, Laudisi F, Sambucci M, Rosado MM. Increased Foxp3+ regulatory T cells in poly (ADP-ribose) polymerase -1 deficiency. J Immunol. 2010; 184:3470–3477.
Article82. Bai P, Hegedus C, Szabo E, Gyure L, Bakondi E, Brunyanszki A, Gergely S, Szabo C, Virag L. Poly ( ADP-Ribose ) polymerase mediates inflammation in a mouse model of contact hypersensitivity. J Invest Dermatol. 2009; 129:234–238.
Article83. Brunyanszki A, Hegedus C, Szanto M, Erdelyi K, Kovacs K, Schreiber V, Gergely S, Kiss B, Szabo E, Virag L, Bai P. Genetic ablation of PARP-1 protects against oxazolone-induced contact hypersensitivity by modulating oxidative stress. J Invest Dermatol. 2010; 130:2629–2637.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study of Susceptibility between Allergic Rhinitis and STAT6 Gene G2964A Polymorphism Study of a Korean Population
- STAT6 Gene Polymorphisms in Allergic Rhinitis
- IL-4 Independent Nuclear Translocalization of STAT6 in HeLa Cells by Entry of Toxoplasma gondii
- IL-13 and STAT6 signaling involve in low dose lipopolysaccharide induced murine model of asthma
- Mast Cells and Allergic Rhinitis