Asia Pac Allergy.
2013 Jul;3(3):194-199. 10.5415/apallergy.2013.3.3.194.
IL-13 and STAT6 signaling involve in low dose lipopolysaccharide induced murine model of asthma
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul 110-799, Korea. guinea71@snu.ac.kr
- 2Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul 110-799, Korea.
- KMID: 2397313
- DOI: http://doi.org/10.5415/apallergy.2013.3.3.194
Abstract
- BACKGROUND
We reported that level of lipopolysaccharide (LPS) exposure determined the type of airway inflammation in a murine model of asthma.
OBJECTIVE
The purpose of this study is to evaluated the role of IL-13 in low dose LPS induced murine model of asthma using IL-13 and signal transducer and activator of transcription 6 (STAT6) deficient mice.
METHODS
Mice were sensitized with an intranasal application of LPS-depleted ovalbumin (OA) and different doses of LPS (0.1 and 10 µg), and then challenged intranasally with OA alone. The phenotype changes between wild type (WT) and IL-13-/- mice and between WT and STAT6-/- mice were evaluated.
RESULTS
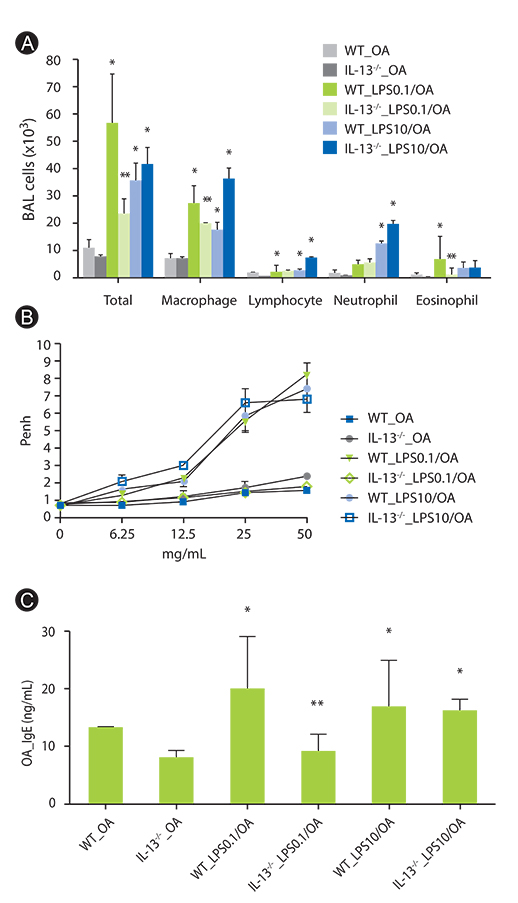
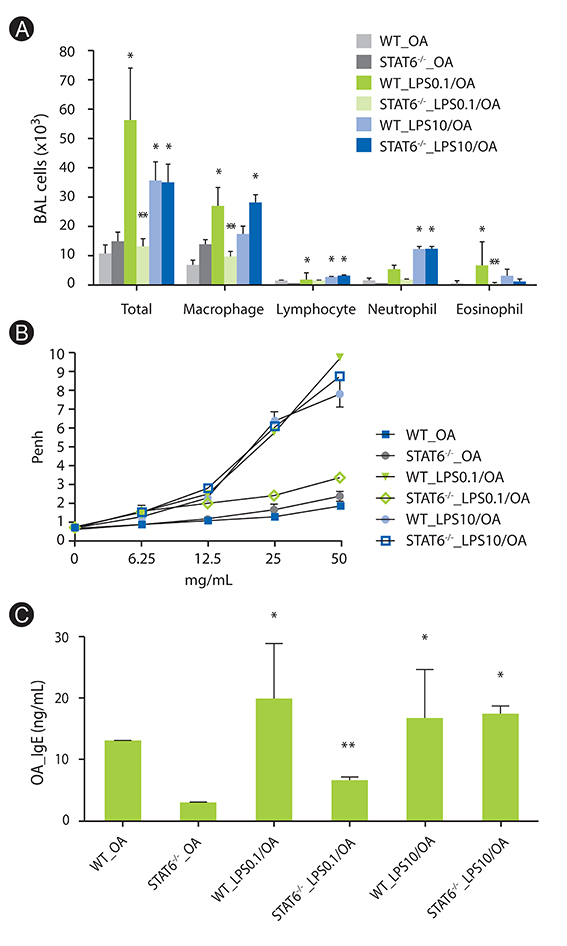
We confirmed again that low and high dose LPS resulted in different phenotypes of murine asthma. In the present study, we observed that phenotypes of murine asthma induced by low dose LPS were abolished in the homozygous null mutation of the IL-13 and STAT6 gene. However, those changes were not shown in mice sensitized OA plus high dose LPS.
CONCLUSION
IL-13 plays an important role in low dose LPS induced murine model of asthma. Our results provided a new insight in understanding of the potential role of IL-13 in innate immunity in human allergic asthma.
Keyword
MeSH Terms
Figure
Reference
-
1. Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Annu Rev Immunol. 1994; 12:295–335.
Article2. Barnes PJ. Pathophysiology of asthma. Br J Clin Pharmacol. 1996; 42:3–10.
Article3. Park JH, Spiegelman DL, Burge HA, Gold DR, Chew GL, Milton DK. Longitudinal study of dust and airborne endotoxin in the home. Environ Health Perspect. 2000; 108:1023–1028.
Article4. Dabbagh K, Dahl ME, Stepick-Biek P, Lewis DB. Toll-like receptor 4 is required for optimal development of Th2 immune responses: role of dendritic cells. J Immunol. 2002; 168:4524–4530.
Article5. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002; 196:1645–1651.
Article6. Wan GH, Li CS, Lin RH. Airborne endotoxin exposure and the development of airway antigen-specific allergic responses. Clin Exp Allergy. 2000; 30:426–432.
Article7. Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, Kim JH, Gho YS, Cho SH, Min KU, Kim YY, Zhu Z. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol. 2007; 178:5375–5382.
Article8. Brightling CE, Saha S, Hollins F. Interleukin-13: prospects for new treatments. Clin Exp Allergy. 2010; 40:42–49.
Article9. Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol. 2000; 105:1063–1070.
Article10. Zurawski G, de Vries JE. Interleukin 13 elicits a subset of the activities of its close relative interleukin 4. Stem Cells. 1994; 12:169–174.
Article11. Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, Leonard WJ. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995; 2:331–339.
Article12. Kumar RK, Foster PS. Modeling allergic asthma in mice: pitfalls and opportunities. Am J Respir Cell Mol Biol. 2002; 27:267–272.13. Kips JC, Anderson GP, Fredberg JJ, Herz U, Inman MD, Jordana M, Kemeny DM, Lötvall J, Pauwels RA, Plopper CG, Schmidt D, Sterk PJ, Van Oosterhout AJ, Vargaftig BB, Chung KF. Murine models of asthma. Eur Respir J. 2003; 22:374–382.
Article14. Zhang Y, Lamm WJ, Albert RK, Chi EY, Henderson WR Jr, Lewis DB. Influence of the route of allergen administration and genetic background on the murine allergic pulmonary response. Am J Respir Crit Care Med. 1997; 155:661–669.
Article15. Chapoval SP, Nabozny GH, Marietta EV, Raymond EL, Krco CJ, Andrews AG, David CS. Short ragweed allergen induces eosinophilic lung disease in HLA-DQ transgenic mice. J Clin Invest. 1999; 103:1707–1717.
Article16. Southam DS, Dolovich M, O'Byrne PM, Inman MD. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002; 282:L833–L839.
Article17. Rylander R, Haglind P, Lundholm M. Endotoxin in cotton dust and respiratory function decrement among cotton workers in an experimental cardroom. Am Rev Respir Dis. 1985; 131:209–213.18. Bonner JC, Rice AB, Lindroos PM, O'Brien PO, Dreher KL, Rosas I, Alfaro-Moreno E, Osornio-Vargas AR. Induction of the lung myofibroblast PDGF receptor system by urban ambient particles from Mexico City. Am J Respir Cell Mol Biol. 1998; 19:672–680.
Article19. Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, Pauwels R, Sergysels R. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996; 154:1641–1646.
Article20. Olenchock SA, May JJ, Pratt DS, Morey PR. Occupational exposures to airborne endotoxins in agriculture. Prog Clin Biol Res. 1987; 231:475–487.21. Berczi I, Bertók L, Bereznai T. Comparative studies on the toxicity of Escherichia coli lipopolysaccharide endotoxin in various animal species. Can J Microbiol. 1966; 12:1070–1071.
Article22. Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998; 282:2261–2263.
Article23. Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol. 2012; 130:829–842.
Article24. Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, Mosesova S, Eisner MD, Bohen SP, Matthews JG. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088–1098.
Article25. Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, She D, Kell C, May RD, Geba GP, Molfino NA. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J. 2013; 41:330–338.
Article26. Hacha J, Tomlinson K, Maertens L, Paulissen G, Rocks N, Foidart JM, Noel A, Palframan R, Gueders M, Cataldo DD. Nebulized anti-IL-13 monoclonal antibody Fab' fragment reduces allergen-induced asthma. Am J Respir Cell Mol Biol. 2012; 47:709–717.
Article27. Wang Y, Li Y, Shan J, Fixman E, McCusker C. Effective treatment of experimental ragweed-induced asthma with STAT-6-IP, a topically delivered cell-penetrating peptide. Clin Exp Allergy. 2011; 41:1622–1630.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- STAT6 and PARP Family Members in the Development of T Cell-dependent Allergic Inflammation
- IL-4 Independent Nuclear Translocalization of STAT6 in HeLa Cells by Entry of Toxoplasma gondii
- IL-4/IL-13 Cytokine and Receptor in Asthma
- STAT6 Expression and IL-13 Production in Association with Goblet Cell Hyperplasia and Worm Expulsion of Gymnophalloides seoi from C57BL/6 Mice
- The Role of IL-17 in a Lipopolysaccharide-Induced Rhinitis Model