Nutr Res Pract.
2016 Jun;10(3):274-281. 10.4162/nrp.2016.10.3.274.
Perilla frutescens var. japonica and rosmarinic acid improve amyloid-β25-35 induced impairment of cognition and memory function
- Affiliations
-
- 1Department of Food Science and Nutrition & Kimchi Research Institute, Pusan National University, 2 Busandaehak-ro 63 beon-gil, Geumjeong-gu, Busan 46241, Korea. ejcho@pusan.ac.kr
- 2Department of Southern Area Crop Science, National Institute of Crop Science, Rural Development Administration, Gyeongnam 50424, Korea.
- 3Department of Integrative Plant Science, Chung-Ang University, Anseong, Gyeonggi 17546, Korea.
- KMID: 2342129
- DOI: http://doi.org/10.4162/nrp.2016.10.3.274
Abstract
- BACKGROUND/OBJECTIVES
The accumulation of amyloid-β (Aβ) in the brain is a hallmark of Alzheimer's disease (AD) and plays a key role in cognitive dysfunction. Perilla frutescens var. japonica extract (PFE) and its major compound, rosmarinic acid (RA), have shown antioxidant and anti-inflammatory activities. We investigated whether administration of PFE and RA contributes to cognitive improvement in an Aβ25-35-injected mouse model.
MATERIALS/METHODS
Male ICR mice were intracerebroventricularly injected with aggregated Aβ25-35 to induce AD. Aβ25-35-injected mice were fed PFE (50 mg/kg/day) or RA (0.25 mg/kg/day) for 14 days and examined for learning and memory ability through the T-maze, object recognition, and Morris water maze test.
RESULTS
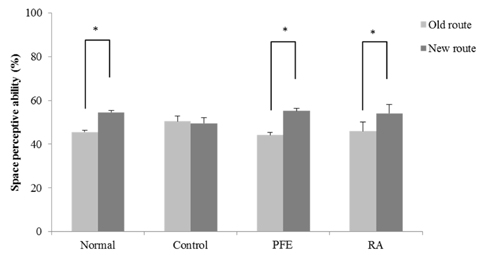
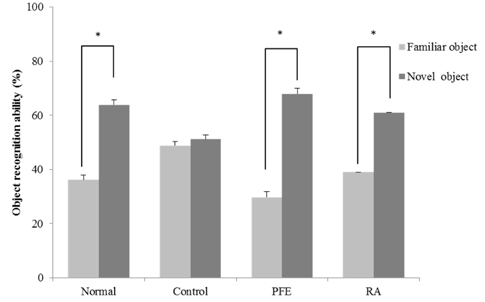
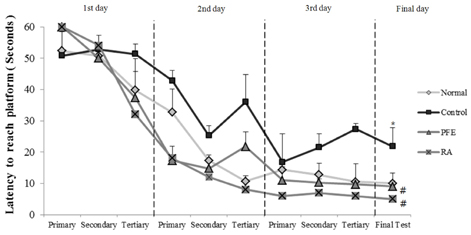
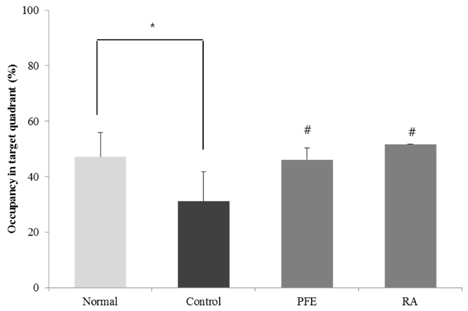
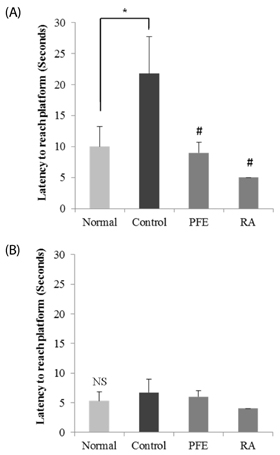
Our present study demonstrated that PFE and RA administration significantly enhanced cognition function and object discrimination, which were impaired by Aβ25-35, in the T-maze and object recognition tests, respectively. In addition, oral administration of PFE and RA decreased the time to reach the platform and increased the number of crossings over the removed platform when compared with the Aβ25-35-induced control group in the Morris water maze test. Furthermore, PFE and RA significantly decreased the levels of nitric oxide (NO) and malondialdehyde (MDA) in the brain, kidney, and liver. In particular, PFE markedly attenuated oxidative stress by inhibiting production of NO and MDA in the Aβ25-35-injected mouse brain.
CONCLUSIONS
These results suggest that PFE and its active compound RA have beneficial effects on cognitive improvement and may help prevent AD induced by Aβ.
MeSH Terms
Figure
Reference
-
1. Collerton D. Cholinergic function and intellectual decline in Alzheimer's disease. Neuroscience. 1986; 19:1–28.
Article2. Golde TE, Eckman CB, Younkin SG. Biochemical detection of Aβ isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer's disease. Biochim Biophys Acta. 2000; 1502:172–187.
Article3. Sambamurti K, Greig NH, Lahiri DK. Advances in the cellular and molecular biology of the beta-amyloid protein in Alzheimer's disease. Neuromolecular Med. 2002; 1:1–31.4. Harman D. Free radical theory of aging: Alzheimer's disease pathogenesis. Age (Omaha). 1995; 18:97–119.
Article5. Götz J, Ittner LM. Animal models of Alzheimer's disease and frontotemporal dementia. Nat Rev Neurosci. 2008; 9:532–544.
Article6. Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS. ABAD enhances Aβ-induced cell stress via mitochondrial dysfunction. FASEB J. 2005; 19:597–598.7. Zussy C, Brureau A, Delair B, Marchal S, Keller E, Ixart G, Naert G, Meunier J, Chevallier N, Maurice T, Givalois L. Time-course and regional analyses of the physiopathological changes induced after cerebral injection of an amyloid β fragment in rats. Am J Pathol. 2011; 179:315–334.
Article8. Honda G, Koezuka Y, Kamisako W, Tabata M. Isolation of sedative principles from Perilla frutescens. Chem Pharm Bull (Tokyo). 1986; 34:1672–1677.
Article9. Kurita N, Koike S. Synergistic antimicrobial effect of sodium chloride and essential oil components. Agric Biol Chem. 1982; 46:159–165.
Article10. Kim MK, Lee HS, Kim EJ, Won NH, Chi YM, Kim BC, Lee KW. Protective effect of aqueous extract of Perilla frutescens on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem Toxicol. 2007; 45:1738–1744.
Article11. Gao LP, Wei HL, Zhao HS, Xiao SY, Zheng RL. Antiapoptotic and antioxidant effects of rosmarinic acid in astrocytes. Pharmazie. 2005; 60:62–65.12. Takano H, Osakabe N, Sanbongi C, Yanagisawa R, Inoue K, Yasuda A, Natsume M, Baba S, Ichiishi E, Yoshikawa T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp Biol Med (Maywood). 2004; 229:247–254.
Article13. Lamaison JL, Petitjean-Freytet C, Carnat A. Rosmarinic acid, total hydroxycinnamic derivatives and antioxidant activity of Apiaceae, Borraginaceae and Lamiceae medicinals. Ann Pharm Fr. 1990; 48:103–108.14. Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996; 706:181–193.
Article15. Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986; 16:355–357.16. Montgomery KC. A test of two explanations of spontaneous alternation. J Comp Physiol Psychol. 1952; 45:287–293.
Article17. Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat Protoc. 2006; 1:1306–1311.
Article18. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11:47–60.
Article19. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.
Article20. Schmidt HH, Warner TD, Nakane M, Förstermann U, Murad F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol. 1992; 41:615–624.21. Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of β-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991; 563:311–314.
Article22. Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990; 250:279–282.
Article23. Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid β-peptide. Trends Mol Med. 2001; 7:548–554.
Article24. Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006; 545:51–64.
Article25. Albarracin SL, Stab B, Casas Z, Sutachan JJ, Samudio I, Gonzalez J, Gonzalo L, Capani F, Morales L, Barreto GE. Effects of natural antioxidants in neurodegenerative disease. Nutr Neurosci. 2012; 15:1–9.
Article26. Takahashi Y. Handbook of Modern Chinese Medicine II. Tokyo: Yakkyoku Shimbun;1969.27. Osakabe N, Yasuda A, Natsume M, Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004; 25:549–557.
Article28. Okuda T, Hatano T, Agata I, Nishibe S. The components of tannic activities in Labiatae plants. I. Rosmarinic acid from Labiatae plants in Japan. Yakugaku Zasshi. 1986; 106:1108–1111.
Article29. Ishikura N. Anthocyanins and flavones in leaves and seeds of Perilla plant. Agric Biol Chem. 1981; 45:1855–1860.
Article30. Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer's beta-amyloid fibrils in vitro. J Neurosci Res. 2004; 75:742–750.
Article31. Makino T, Ono T, Matsuyama K, Nogaki F, Miyawaki S, Honda G, Muso E. Suppressive effects of Perilla frutescens on IgA nephropathy in HIGA mice. Nephrol Dial Transplant. 2003; 18:484–490.
Article32. Alkam T, Nitta A, Mizoguchi H, Itoh A, Nabeshima T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Aβ(25-35). Behav Brain Res. 2007; 180:139–145.
Article33. Pereira P, Tysca D, Oliveira P, da Silva Brum LF, Picada JN, Ardenghi P. Neurobehavioral and genotoxic aspects of rosmarinic acid. Pharmacol Res. 2005; 52:199–203.
Article34. Murai T, Okuda S, Tanaka T, Ohta H. Characteristics of object location memory in mice: behavioral and pharmacological studies. Physiol Behav. 2007; 90:116–124.
Article35. Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci U S A. 1999; 96:3228–3233.
Article36. Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006; 443:768–773.
Article37. Baluchnejadmojarad T, Roghani M, Homayounfar H. Inhibitory effect of high dose of the flavonoid quercetin on amygdala electrical kindling in rats. Basic Clin Neurosci. 2010; 1:57–61.38. Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002; 32:1050–1060.
Article39. Cecchi C, Fiorillo C, Baglioni S, Pensalfini A, Bagnoli S, Nacmias B, Sorbi S, Nosi D, Relini A, Liguri G. Increased susceptibility to amyloid toxicity in familial Alzheimer's fibroblasts. Neurobiol Aging. 2007; 28:863–876.
Article40. Palmer AM, Burns MA. Selective increase in lipid peroxidation in the inferior temporal cortex in Alzheimer's disease. Brain Res. 1994; 645:338–342.
Article41. Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer's disease. Biomark Med. 2010; 4:27–36.
Article42. Mattson MP, Begley JG, Mark RJ, Furukawa K. Aβ25-35 induces rapid lysis of red blood cells: contrast with Aβ1-42 and examination of underlying mechanisms. Brain Res. 1997; 771:147–153.
Article43. Smith MA, Sayre LM, Monnier VM, Perry G. Radical ageing in Alzheimer's disease. Trends Neurosci. 1995; 18:172–176.
Article44. Choi JY, Cho EJ, Lee HS, Lee JM, Yoon YH, Lee S. Tartary buckwheat improves cognition and memory function in an in vivo amyloid-β-induced Alzheimer model. Food Chem Toxicol. 2013; 53:105–111.
Article45. Choi JY, Lee JM, Lee DG, Cho S, Yoon YH, Cho EJ, Lee S. The n-butanol fraction and rutin from tartary buckwheat improve cognition and memory in an in vivo model of amyloid-β-induced Alzheimer's disease. J Med Food. 2015; 18:631–641.
Article46. Lee AY, Yamabe N, Kang KS, Kim HY, Lee S, Cho EJ. Cognition and memory function of Taraxacum coreanum in an in vivo amyloid-β-induced mouse model of Alzheimer's disease. Arch Biol Sci. 2014; 66:1357–1366.
Article47. Schirrmacher G, Skurk T, Hauner H, Grassmann J. Effect of Spinacia oleraceae L. and Perilla frutescens L. on antioxidants and lipid peroxidation in an intervention study in healthy individuals. Plant Foods Hum Nutr. 2010; 65:71–76.
Article48. Iuvone T, De Filippis D, Esposito G, D'Amico A, Izzo AA. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-β peptide-induced neurotoxicity. J Pharmacol Exp Ther. 2006; 317:1143–1149.
Article49. Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996; 271:C1424–C1437.
Article50. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010; 37:13–25.
Article51. Jancsó G, Domoki F, Sántha P, Varga J, Fischer J, Orosz K, Penke B, Becskei A, Dux M, Tóth L. β-Amyloid (1-42) peptide impairs blood-brain barrier function after intracarotid infusion in rats. Neurosci Lett. 1998; 253:139–141.
Article52. Schwab C, McGeer PL. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J Alzheimers Dis. 2008; 13:359–369.
Article53. Falé PL, Madeira PJ, Florêncio MH, Ascensão L, Serralheiro ML. Function of Plectranthus barbatus herbal tea as neuronal acetylcholinesterase inhibitor. Food Funct. 2011; 2:130–136.
Article54. Lebrun C, Pillière E, Lestage P. Effects of S 18986-1, a novel cognitive enhancer, on memory performances in an object recognition task in rats. Eur J Pharmacol. 2000; 401:205–212.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Neuro-Protective Effect of the Methanolic Extract of Perilla frutescens var. japonica and Rosmarinic Acid against H2O2-Induced Oxidative Stress in C6 Glial Cells
- Fatty Acid Content in Perilla Cultivars and Commercial Oils Determined by GC Analysis
- Anti-Helicobacter pylori activity of acomplex mixture of Lactobacillus paracasei HP7 including the extract of Perilla frutescens var. acuta and Glycyrrhiza glabra
- Korean Mistletoe (Viscum album var. coloratum) Inhibits Amyloid beta Protein (25-35)-induced Cultured Neuronal Cell Damage and Memory Impairment
- Inhibition of Proinflammatory Cytokine Generation in Lung Inflammation by the Leaves of Perilla frutescens and Its Constituents