Nutr Res Pract.
2016 Jun;10(3):259-264. 10.4162/nrp.2016.10.3.259.
Compound K attenuates stromal cell-derived growth factor 1 (SDF-1)-induced migration of C6 glioma cells
- Affiliations
-
- 1Department of Diagnostics, College of Korean Medicine, Dongguk University, Goyang 10326, Korea.
- 2Department of Acupoint, College of Korean Medicine, Dongguk University, Dongguk-Ro 32, Goyang 10326, Korea. amppaper@dongguk.ac.kr
- 3Department of Pathology, College of Korean Medicine, Dongguk University, Goyang 10326, Korea.
- 4Department of Prescription, College of Korean Medicine, Dongguk University, Goyang 10326, Korea.
- KMID: 2342127
- DOI: http://doi.org/10.4162/nrp.2016.10.3.259
Abstract
- BACKGROUND/OBJECTIVES
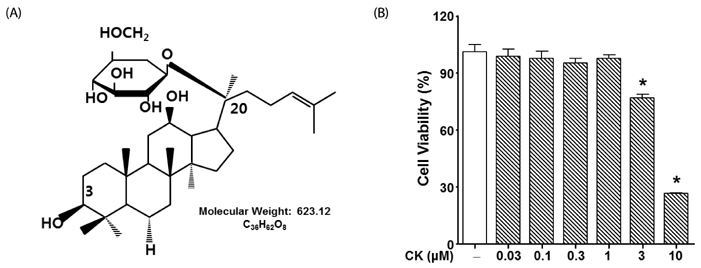
Stromal cell-derived growth factor 1 (SDF-1), also known as chemokine ligand 12, and chemokine receptor type 4 are involved in cancer cell migration. Compound K (CK), a metabolite of protopanaxadiol-type ginsenoside by gut microbiota, is reported to have therapeutic potential in cancer therapy. However, the inhibitory effect of CK on SDF-1 pathway-induced migration of glioma has not yet been established.
MATERIALS/METHODS
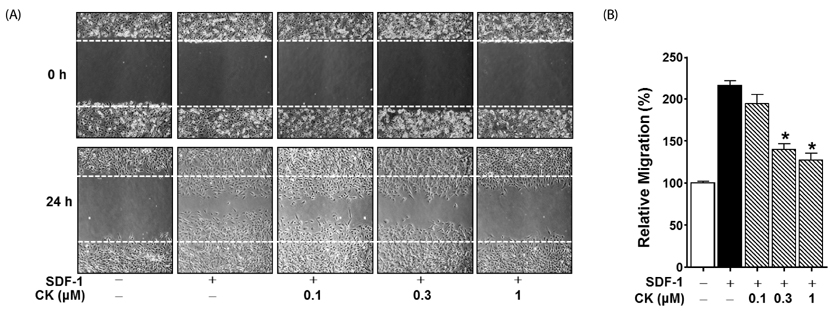
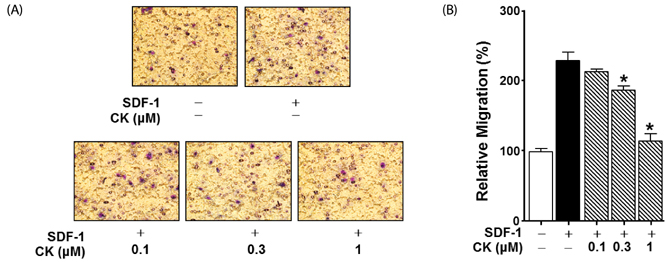
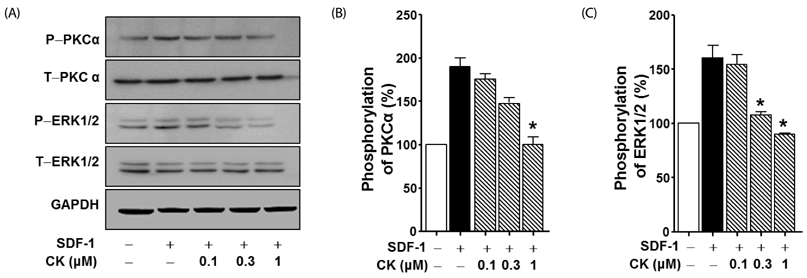
Cytotoxicity of CK in C6 glioma cells was determined using an EZ-Cytox cell viability assay kit. Cell migration was tested using the wound healing and Boyden chamber assay. Phosphorylation levels of protein kinase C (PKC)α and extracellular signal-regulated kinase (ERK) were measured by western blot assay, and matrix metallopeptidases (MMP) were measured by gelatin-zymography analysis.
RESULTS
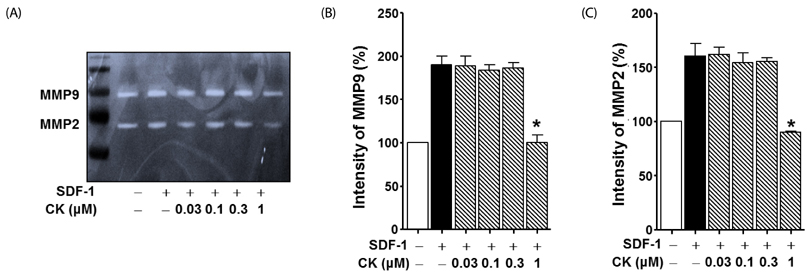
CK significantly reduced the phosphorylation of PKCα and ERK1/2, expression of MMP9 and MMP2, and inhibited the migration of C6 glioma cells under SDF-1-stimulated conditions.
CONCLUSIONS
CK is a cell migration inhibitor that inhibits C6 glioma cell migration by regulating its downstream signaling molecules including PKCα, ERK1/2, and MMPs.
Keyword
MeSH Terms
Figure
Reference
-
1. Amberger VR, Hensel T, Ogata N, Schwab ME. Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res. 1998; 58:149–158.2. Chintala SK, Rao JK. Invasion of human glioma: role of extracellular matrix proteins. Front Biosci. 1996; 1:d324–d339.
Article3. Stock AM, Hahn SA, Troost G, Niggemann B, Zänker KS, Entschladen F. Induction of pancreatic cancer cell migration by an autocrine epidermal growth factor receptor activation. Exp Cell Res. 2014; 326:307–314.
Article4. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013; 14:e218–e228.
Article5. Bajetto A, Barbieri F, Dorcaratto A, Barbero S, Daga A, Porcile C, Ravetti JL, Zona G, Spaziante R, Corte G, Schettini G, Florio T. Expression of CXC chemokine receptors 1-5 and their ligands in human glioma tissues: role of CXCR4 and SDF1 in glioma cell proliferation and migration. Neurochem Int. 2006; 49:423–432.
Article6. Di Cesare S, Marshall JC, Fernandes BF, Logan P, Antecka E, Filho VB, Burnier MN Jr. In vitro characterization and inhibition of the CXCR4/CXCL12 chemokine axis in human uveal melanoma cell lines. Cancer Cell Int. 2007; 7:17.
Article7. Heckmann D, Maier P, Laufs S, Wenz F, Zeller WJ, Fruehauf S, Allgayer H. CXCR4 expression and treatment with SDF-1α or Plerixafor modulate proliferation and chemosensitivity of colon cancer cells. Transl Oncol. 2013; 6:124–132.
Article8. Luker KE, Lewin SA, Mihalko LA, Schmidt BT, Winkler JS, Coggins NL, Thomas DG, Luker GD. Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells. Oncogene. 2012; 31:4750–4758.
Article9. Ray P, Lewin SA, Mihalko LA, Schmidt BT, Luker KE, Luker GD. Noninvasive imaging reveals inhibition of ovarian cancer by targeting CXCL12-CXCR4. Neoplasia. 2011; 13:1152–1161.
Article10. Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007; 256:137–165.
Article11. Iwasa S, Yanagawa T, Fan J, Katoh R. Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric cancer is associated with lymph node and liver metastasis. Anticancer Res. 2009; 29:4751–4758.12. Lahn MM, Sundell KL, Paterson BM. The role of protein kinase C-alpha in malignancies of the nervous system and implications for the clinical development of the specific PKC-alpha inhibitor aprinocarsen (Review). Oncol Rep. 2004; 11:515–522.13. Koivunen J, Aaltonen V, Peltonen J. Protein kinase C (PKC) family in cancer progression. Cancer Lett. 2006; 235:1–10.
Article14. Troppmair J, Bruder JT, Munoz H, Lloyd PA, Kyriakis J, Banerjee P, Avruch J, Rapp UR. Mitogen-activated protein kinase/extracellular signal-regulated protein kinase activation by oncogenes, serum, and 12-O-tetradecanoylphorbol-13-acetate requires Raf and is necessary for transformation. J Biol Chem. 1994; 269:7030–7035.
Article15. Lee DC, Lau AS. Effects of Panax ginseng on tumor necrosis factor-α -mediated inflammation: a mini-review. Molecules. 2011; 16:2802–2816.
Article16. Paul S, Shin HS, Kang SC. Inhibition of inflammations and macrophage activation by ginsenoside-Re isolated from Korean ginseng (Panax ginseng C.A. Meyer). Food Chem Toxicol. 2012; 50:1354–1361.
Article17. Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Di Buono M, Lee KS, Leiter LA, Nam KY, Arnason JT, Choi M, Naeem A. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008; 18:46–56.
Article18. Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J Nutr. 2007; 137:183S–185S.
Article19. Yuan CS, Wang CZ, Wicks SM, Qi LW. Chemical and pharmacological studies of saponins with a focus on American ginseng. J Ginseng Res. 2010; 34:160–167.
Article20. Qi LW, Wang CZ, Du GJ, Zhang ZY, Calway T, Yuan CS. Metabolism of ginseng and its interactions with drugs. Curr Drug Metab. 2011; 12:818–822.
Article21. Chen Y, Xu Y, Zhu Y, Li X. Anti-cancer effects of ginsenoside compound k on pediatric acute myeloid leukemia cells. Cancer Cell Int. 2013; 13:24.
Article22. Wang CZ, Kim KE, Du GJ, Qi LW, Wen XD, Li P, Bauer BA, Bissonnette MB, Musch MW, Chang EB, Yuan CS. Ultra-performance liquid chromatography and time-of-flight mass spectrometry analysis of ginsenoside metabolites in human plasma. Am J Chin Med. 2011; 39:1161–1171.
Article23. Song G, Guo S, Wang W, Hu C, Mao Y, Zhang B, Zhang H, Hu T. Intestinal metabolite compound K of ginseng saponin potently attenuates metastatic growth of hepatocellular carcinoma by augmenting apoptosis via a Bid-mediated mitochondrial pathway. J Agric Food Chem. 2010; 58:12753–12760.
Article24. Chae S, Kang KA, Chang WY, Kim MJ, Lee SJ, Lee YS, Kim HS, Kim DH, Hyun JW. Effect of compound K, a metabolite of ginseng saponin, combined with gamma-ray radiation in human lung cancer cells in vitro and in vivo. J Agric Food Chem. 2009; 57:5777–5782.
Article25. Lee KP, Choi NH, Kim JT, Park IS. The effect of yacon (Samallanthus sonchifolius) ethanol extract on cell proliferation and migration of C6 glioma cells stimulated with fetal bovine serum. Nutr Res Pract. 2015; 9:256–261.
Article26. Kobayashi T, Hattori S, Shinkai H. Matrix metalloproteinases-2 and -9 are secreted from human fibroblasts. Acta Derm Venereol. 2003; 83:105–107.
Article27. Chinot OL, Macdonald DR, Abrey LE, Zahlmann G, Kerloëguen Y, Cloughesy TF. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep. 2013; 13:347.
Article28. Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977-2000. Cancer. 2004; 101:2293–2299.
Article29. Paek IB, Moon Y, Kim J, Ji HY, Kim SA, Sohn DH, Kim JB, Lee HS. Pharmacokinetics of a ginseng saponin metabolite compound K in rats. Biopharm Drug Dispos. 2006; 27:39–45.
Article30. Valenzuela-Fernández A, Planchenault T, Baleux F, Staropoli I, Le-Barillec K, Leduc D, Delaunay T, Lazarini F, Virelizier JL, Chignard M, Pidard D, Arenzana-Seisdedos F. Leukocyte elastase negatively regulates Stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002; 277:15677–15689.
Article31. Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006; 107:1761–1767.
Article32. Hattermann K, Mentlein R. An infernal trio: the chemokine CXCL12 and its receptors CXCR4 and CXCR7 in tumor biology. Ann Anat. 2013; 195:103–110.
Article33. Mukherjee D, Zhao J. The role of chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer Res. 2013; 3:46–57.34. Ehtesham M, Min E, Issar NM, Kasl RA, Khan IS, Thompson RC. The role of the CXCR4 cell surface chemokine receptor in glioma biology. J Neurooncol. 2013; 113:153–162.
Article35. do Carmo A, Patricio I, Cruz MT, Carvalheiro H, Oliveira CR, Lopes MC. CXCL12/CXCR4 promotes motility and proliferation of glioma cells. Cancer Biol Ther. 2010; 9:56–65.
Article36. Jung SH, Woo MS, Kim SY, Kim WK, Hyun JW, Kim EJ, Kim DH, Kim HS. Ginseng saponin metabolite suppresses phorbol ester-induced matrix metalloproteinase-9 expression through inhibition of activator protein-1 and mitogen-activated protein kinase signaling pathways in human astroglioma cells. Int J Cancer. 2006; 118:490–497.
Article37. Fang W, Li H, Kong L, Niu G, Gao Q, Zhou K, Zheng J, Wu B. Role of matrix metalloproteinases (MMPs) in tumor invasion and metastasis: serial studies on MMPs and TIMPs. Beijing Da Xue Xue Bao. 2003; 35:441–443.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stromal Cell-Derived Factor-1 Promotes Myeloma Cell Growth in Both Autocrine and Paracrine Manners
- Cytoplasmic Trapping of CXCR4 in Hepatocellular Carcinoma Cell Lines
- Regulation of Adhesion Molecule Expression and Stromal Cell-Derived Factor-1 Production in Human Bone Marrow Cells by Interferon-gamma, Tumor Necrosis Factor-alpha, and Transforming Growth Factor-beta1: Implications in Bone Marrow Homing of Hematopoietic Cells
- Production of Stromal Cell-Derived Factor-1 (SDF-1)and Expression of CXCR4 in Human Bone Marrow Endothelial Cells
- Effect of Tamoxifen in C6 Glioma Cells