J Korean Endocr Soc.
2008 Aug;23(4):238-244.
Effects of Simvastatin on the Growth and Invasion of Anaplastic Thyroid Cancer Cells Lines
- Affiliations
-
- 1Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Korea.
- 2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Korea.
Abstract
- BACKGROUND
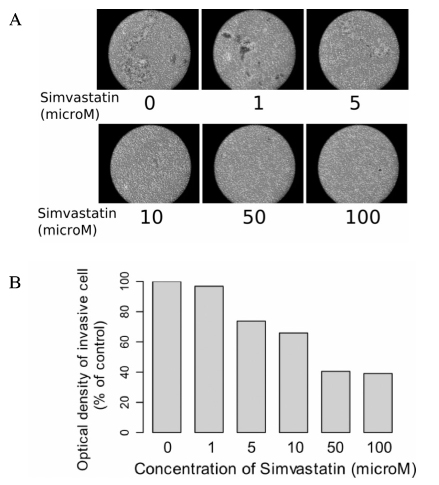
Anaplastic thyroid carcinoma has grave prognosis with most patient dying within 6 months of diagnosis. 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors have been reported to have an anticancer effect in experimental and clinical studies. In this study, we investigated the effect of HMG-CoA reductase inhibitors on cell growth, invasiveness, adherence and signal transduction to evaluate the possibility of simvastatin as an agent for treatment of thyroid cancer. METHODS: The viability of simvastatin treated 3 thyroid cancer cell lines (FRO, WRO, and ARO) were determined. We evaluated the cell migration, anchorage-independent growth and invasion ability in anaplastic thyroid cell line. The expression and phosphorylation of focal adhesion kinase (FAK) and extracellular signal-regurated kinase (ERK) were determined by immunoblot analysis. RESULTS: Three thyroid cancer cell lines showed concentration dependent decrease of viability after treatment with 100~200 mM of simvastatin. Anaplastic ARO cell line showed the most predominant decrease in viability. In ARO cell lines, cell migration was decreased by concentration dependent manner after treatment with simvastatin (concentration > or = 5 mM). Anchorage independent colony formation also decreased after simvastatin (> or = 10 mM). Finally, immunoblot analysis revealed that the phosphorylation status of FAK and ERK decreased in time dependent manner following treatment with 10 mM of simvastatin. CONCLUSION: The results of this study suggest that simvstatin exerts a favorable effect on the progression and metastasis of thyroid cancer. However, further studies are needed to elucidate the related mechanisms and signal transductions prior to its therapeutic application.
MeSH Terms
-
Acyl Coenzyme A
Cell Line
Cell Movement
Focal Adhesion Protein-Tyrosine Kinases
Humans
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Neoplasm Metastasis
Oxidoreductases
Phosphorylation
Phosphotransferases
Prognosis
Signal Transduction
Simvastatin
Thyroid Gland
Thyroid Neoplasms
Acyl Coenzyme A
Focal Adhesion Protein-Tyrosine Kinases
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Oxidoreductases
Phosphotransferases
Simvastatin
Thyroid Neoplasms
Figure
Reference
-
1. Pasieka JL. Anaplastic thyroid cancer. Curr Opin Oncol. 2003. 15:78–83.2. Kim TY, Kim KW, Jung TS, Kim JM, Kim SW, Chung KW, Kim EY, Gong G, Oh YL, Cho SY, Yi KH, Kim WB, Park do J, Chung JH, Cho BY, Shong YK. Prognostic factors for Korean patients with anaplastic thyroid carcinoma. Head Neck. 2007. 29:765–772.3. Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996. 7:1209–1224.4. Richardson A, Parsons T. A mechanism for regulation of the adhesion-associated proteintyrosine kinase pp125FAK. Nature. 1996. 380:538–540.5. Sanders MA, Basson MD. Collagen IV-dependent ERK activation in human Caco-2 intestinal epithelial cells requires focal adhesion kinase. J Biol Chem. 2000. 275:38040–38047.6. Crowe DL, Ohannessian A. Recruitment of focal adhesion kinase and paxillin to beta1 integrin promotes cancer cell migration via mitogen activated protein kinase activation. BMC Cancer. 2004. 4:18.7. Sawai H, Okada Y, Funahashi H, Matsuo Y, Takahashi H, Takeyama H, Manabe T. Activation of focal adhesion kinase enhances the adhesion and invasion of pancreatic cancer cells via extracellular signal-regulated kinase-1/2 signaling pathway activation. Mol Cancer. 2005. 4:37.8. Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, Akedo H, Inoue M, Nakamura H. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology. 2002. 122:308–317.9. Lennernas H, Fager G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Similarities and differences. Clin Pharmacokinet. 1997. 32:403–425.10. Welch HG. Statins and the risk of colorectal cancer. N Engl J Med. 2005. 353:952–954.11. Yasunari K, Maeda K, Minami M, Yoshikawa J. HMG-CoA reductase inhibitors prevent migration of human coronary smooth muscle cells through suppression of increase in oxidative stress. Arterioscler Thromb Vasc Biol. 2001. 21:937–942.12. Farina HG, Bublik DR, Alonso DF, Gomez DE. Lovastatin alters cytoskeleton organization and inhibits experimental metastasis of mammary carcinoma cells. Clin Exp Metastasis. 2002. 19:551–559.13. Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, Akedo H, Nakamura H. Inhibition of epidermal growth factor-induced RhoA translocation and invasion of human pancreatic cancer cells by 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. Cancer Res. 2001. 61:4885–4891.14. Koyuturk M, Ersoz M, Altiok N. Simvastatin induces apoptosis in human breast cancer cells: p53 and estrogen receptor independent pathway requiring signalling through JNK. Cancer Lett. 2007. 250:220–228.15. Takeda I, Maruya S, Shirasaki T, Mizukami H, Takahata T, Myers JN, Kakehata S, Yagihashi S, Shinkawa H. Simvastatin inactivates beta1-integrin and extracellular signal-related kinase signaling and inhibits cell proliferation in head and neck squamous cell carcinoma cells. Cancer Sci. 2007. 98:890–899.16. Zhong WB, Liang YC, Wang CY, Chang TC, Lee WS. Lovastatin suppresses invasiveness of anaplastic thyroid cancer cells by inhibiting Rho geranylgeranylation and RhoA/ROCK signaling. Endocr Relat Cancer. 2005. 12:615–629.17. Kim WS, Kim MM, Choi HJ, Yoon SS, Lee MH, Park K, Park CH, Kang WK. Phase II study of high-dose lovastatin in patients with advanced gastric adenocarcinoma. Invest New Drugs. 2001. 19:81–83.18. Larner J, Jane J, Laws E, Packer R, Myers C, Shaffrey M. A phase I-II trial of lovastatin for anaplastic astrocytoma and glioblastoma multiforme. Am J Clin Oncol. 1998. 21:579–583.19. Kawata S, Yamasaki E, Nagase T, Inui Y, Ito N, Matsuda Y, Inada M, Tamura S, Noda S, Imai Y, Matsuzawa Y. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer. 2001. 84:886–891.20. Cappelli C, Castellano M, Pirola I, De Martino E, Gandossi E, Delbarba A, Salvi A, Rosei EA. Reduced thyroid volume and nodularity in dyslipidaemic patients on statin treatment. Clin Endocrinol (Oxf). 2008. 68:16–21.21. Backman JT, Kyrklund C, Kivisto KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000. 68:122–129.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Berberine Inhibited the Growth of Thyroid Cancer Cell Lines 8505C and TPC1
- Aberrant L1 Cell Adhesion Molecule Expression in Cancer: In View of Proliferation, Migration and Invasion
- Role of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside in the Growth Regulation of Anaplastic Thyroid Cancer Cells Lines
- Treatment Effect of Combining Lenvatinib and Vemurafenib for BRAF Mutated Anaplastic Thyroid Cancer
- Effects of Peroxisome Proliferator-Activated Receptor (PPAR) Delta on the Growth and Invasion of a Thyroid Cancer Cell Line