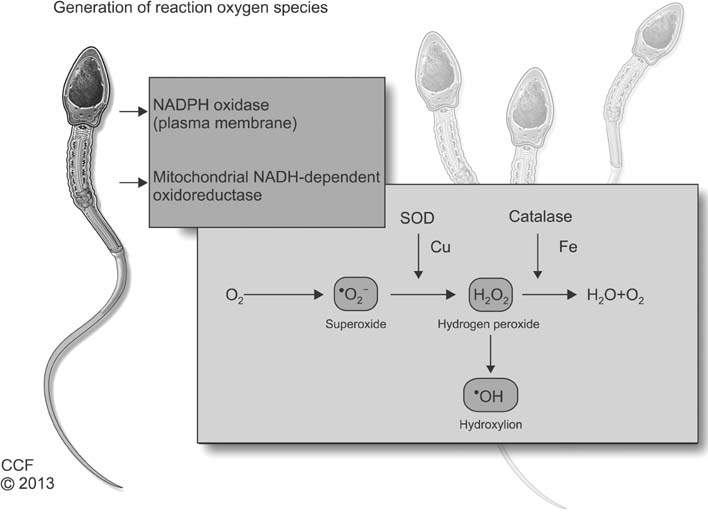
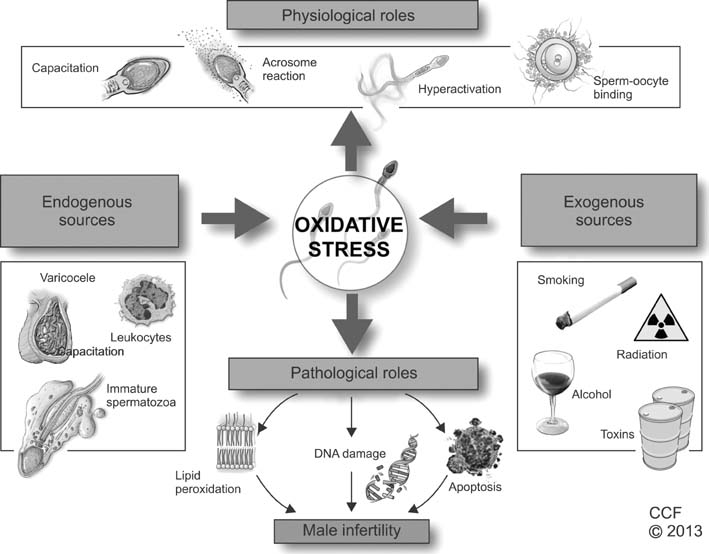
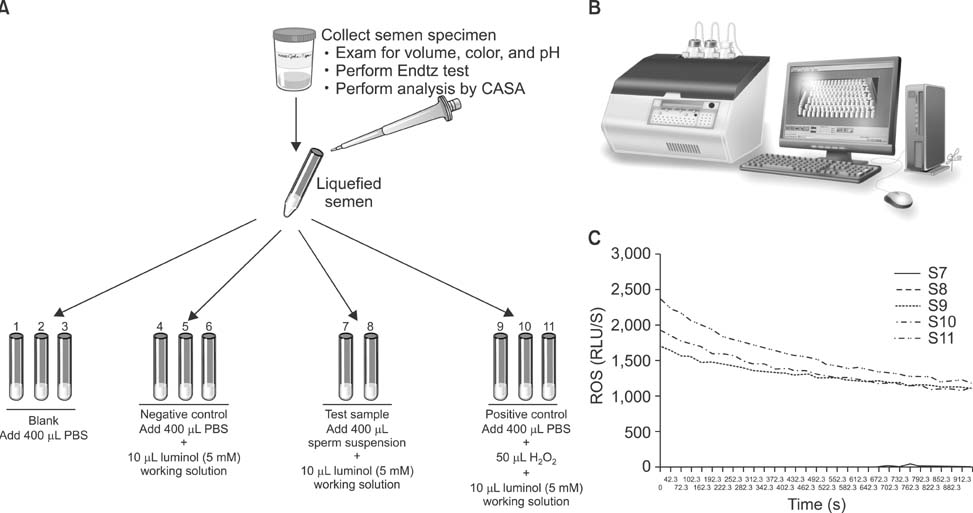
World J Mens Health.
2014 Apr;32(1):1-17.
Effect of Oxidative Stress on Male Reproduction
- Affiliations
-
- 1Center for Reproductive Medicine, Cleveland Clinic, Cleveland, OH, USA. agarwaa@ccf.org
- 2Medical Physiology, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg, South Africa.
Abstract
- Infertility affects approximately 15% of couples trying to conceive, and a male factor contributes to roughly half of these cases. Oxidative stress (OS) has been identified as one of the many mediators of male infertility by causing sperm dysfunction. OS is a state related to increased cellular damage triggered by oxygen and oxygen-derived free radicals known as reactive oxygen species (ROS). During this process, augmented production of ROS overwhelms the body's antioxidant defenses. While small amounts of ROS are required for normal sperm functioning, disproportionate levels can negatively impact the quality of spermatozoa and impair their overall fertilizing capacity. OS has been identified as an area of great attention because ROS and their metabolites can attack DNA, lipids, and proteins; alter enzymatic systems; produce irreparable alterations; cause cell death; and ultimately, lead to a decline in the semen parameters associated with male infertility. This review highlights the mechanisms of ROS production, the physiological and pathophysiological roles of ROS in relation to the male reproductive system, and recent advances in diagnostic methods; it also explores the benefits of using antioxidants in a clinical setting.
MeSH Terms
Figure
Reference
-
1. Trussell JC. Optimal diagnosis and medical treatment of male infertility. Semin Reprod Med. 2013; 31:235–236.
Article2. World Health Organisation. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization;2010.3. Agarwal A, Sekhon LH. Oxidative stress and antioxidants for idiopathic oligoasthenoteratospermia: is it justified? Indian J Urol. 2011; 27:74–85.
Article4. Saalu LC. The incriminating role of reactive oxygen species in idiopathic male infertility: an evidence based evaluation. Pak J Biol Sci. 2010; 13:413–422.
Article5. Hampl R, Drábková P, Kanďár R, Stěpán J. Impact of oxidative stress on male infertility. Ceska Gynekol. 2012; 77:241–245.6. Henkel RR. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl. 2011; 13:43–52.
Article7. Lanzafame FM, La Vignera S, Vicari E, Calogero AE. Oxidative stress and medical antioxidant treatment in male infertility. Reprod Biomed Online. 2009; 19:638–659.
Article8. Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl. 2002; 23:737–752.9. Bansal AK, Bilaspuri GS. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int. 2010; 2010:686137.
Article10. Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011; 26:1628–1640.
Article11. Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update. 2008; 14:243–258.
Article12. De Iuliis GN, Wingate JK, Koppers AJ, McLaughlin EA, Aitken RJ. Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. J Clin Endocrinol Metab. 2006; 91:1968–1975.
Article13. Aitken RJ, Baker MA, De Iuliis GN, Nixon B. New insights into sperm physiology and pathology. Handb Exp Pharmacol. 2010; (198):99–115.
Article14. Butler A, He X, Gordon RE, Wu HS, Gatt S, Schuchman EH. Reproductive pathology and sperm physiology in acid sphingomyelinase-deficient mice. Am J Pathol. 2002; 161:1061–1075.
Article15. Miranda-Vilela AL, Alves PC, Akimoto AK, Pereira LC, Nazaré Klautau-Guimarães M, Grisolia CK. The effect of hydrogen peroxide-induced oxidative stress on leukocytes depends on age and physical training in healthy human subjects carrying the same genotypes of antioxidant enzymes' gene polymorphisms. Am J Hum Biol. 2010; 22:807–812.
Article16. Chen SJ, Allam JP, Duan YG, Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet. 2013; 288:191–199.
Article17. Hazout A, Menezo Y, Madelenat P, Yazbeck C, Selva J, Cohen-Bacrie P. Causes and clinical implications of sperm DNA damages. Gynecol Obstet Fertil. 2008; 36:1109–1117.18. Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem. 2001; 8:851–862.
Article19. Sabeur K, Ball BA. Characterization of NADPH oxidase 5 in equine testis and spermatozoa. Reproduction. 2007; 134:263–270.
Article20. Choudhary R, Chawala VK, Soni ND, Kumar J, Vyas RK. Oxidative stress and role of antioxidants in male infertility. Pak J Physiol. 2010; 6:54–59.21. Esteves SC. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Int Braz J Urol. 2002; 28:484–485.22. Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 2003; 79:Suppl 3. 1597–1605.
Article23. Lavranos G, Balla M, Tzortzopoulou A, Syriou V, Angelopoulou R. Investigating ROS sources in male infertility: a common end for numerous pathways. Reprod Toxicol. 2012; 34:298–307.
Article24. Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003; 79:829–843.
Article25. Lu JC, Huang YF, Lü NQ. WHO Laboratory Manual for the Examination and Processing of Human Semen: its applicability to andrology laboratories in China. Zhonghua Nan Ke Xue. 2010; 16:867–871.26. Nandipati KC, Pasqualotto FF, Thomas AJ Jr, Agarwal A. Relationship of interleukin-6 with semen characteristics and oxidative stress in vasectomy reversal patients. Andrologia. 2005; 37:131–134.
Article27. Rengan AK, Agarwal A, van der Linde M, du Plessis SS. An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reprod Biol Endocrinol. 2012; 10:92.
Article28. Will MA, Swain J, Fode M, Sonksen J, Christman GM, Ohl D. The great debate: varicocele treatment and impact on fertility. Fertil Steril. 2011; 95:841–852.
Article29. Shiraishi K, Matsuyama H, Takihara H. Pathophysiology of varicocele in male infertility in the era of assisted reproductive technology. Int J Urol. 2012; 19:538–550.
Article30. Agarwal A, Deepinder F, Sharma RK, Ranga G, Li J. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril. 2008; 89:124–128.
Article31. Aitken RJ, Bennetts LE, Sawyer D, Wiklendt AM, King BV. Impact of radio frequency electromagnetic radiation on DNA integrity in the male germline. Int J Androl. 2005; 28:171–179.
Article32. De Iuliis GN, Newey RJ, King BV, Aitken RJ. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS One. 2009; 4:e6446.
Article33. Esfandiari N, Saleh RA, Blaut AP, Sharma RK, Nelson DR, Thomas AJ Jr, et al. Effects of temperature on sperm motion characteristics and reactive oxygen species. Int J Fertil Womens Med. 2002; 47:227–233.34. Pant N, Shukla M, Kumar Patel D, Shukla Y, Mathur N, Kumar Gupta Y, et al. Correlation of phthalate exposures with semen quality. Toxicol Appl Pharmacol. 2008; 231:112–116.
Article35. Latini G, Del Vecchio A, Massaro M, Verrotti A, De Felice C. Phthalate exposure and male infertility. Toxicology. 2006; 226:90–98.
Article36. Kasahara E, Sato EF, Miyoshi M, Konaka R, Hiramoto K, Sasaki J, et al. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem J. 2002; 365:849–856.
Article37. Jurasović J, Cvitković P, Pizent A, Colak B, Telisman S. Semen quality and reproductive endocrine function with regard to blood cadmium in Croatian male subjects. Biometals. 2004; 17:735–743.
Article38. Saleh RA, Agarwal A, Sharma RK, Nelson DR, Thomas AJ Jr. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertil Steril. 2002; 78:491–499.
Article39. Jarow JP. Semen quality of male smokers and nonsmokers in infertile couples. J Urol. 2003; 170:675–676.40. Kiziler AR, Aydemir B, Onaran I, Alici B, Ozkara H, Gulyasar T, et al. High levels of cadmium and lead in seminal fluid and blood of smoking men are associated with high oxidative stress and damage in infertile subjects. Biol Trace Elem Res. 2007; 120:82–91.
Article41. Agarwal A, Prabakaran SA. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005; 43:963–974.42. Tsai WW, Niessen S, Goebel N, Yates JR 3rd, Guccione E, Montminy M. PRMT5 modulates the metabolic response to fasting signals. Proc Natl Acad Sci U S A. 2013; 110:8870–8875.
Article43. Kothari S, Thompson A, Agarwal A, du Plessis SS. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol. 2010; 48:425–435.44. de Lamirande E, O'Flaherty C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta. 2008; 1784:106–115.
Article45. Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008; 14:647–657.
Article46. Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009; 129:357–367.47. Calamera J, Buffone M, Ollero M, Alvarez J, Doncel GF. Superoxide dismutase content and fatty acid composition in subsets of human spermatozoa from normozoospermic, asthenozoospermic, and polyzoospermic semen samples. Mol Reprod Dev. 2003; 66:422–430.
Article48. Khosrowbeygi A, Zarghami N. Fatty acid composition of human spermatozoa and seminal plasma levels of oxidative stress biomarkers in subfertile males. Prostaglandins Leukot Essent Fatty Acids. 2007; 77:117–121.
Article49. Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2004; 2:12.50. Zribi N, Chakroun NF, Elleuch H, Abdallah FB, Ben Hamida AS, Gargouri J, et al. Sperm DNA fragmentation and oxidation are independent of malondialdheyde. Reprod Biol Endocrinol. 2011; 9:47.
Article51. Schulte RT, Ohl DA, Sigman M, Smith GD. Sperm DNA damage in male infertility: etiologies, assays, and outcomes. J Assist Reprod Genet. 2010; 27:3–12.
Article52. Kemal Duru N, Morshedi M, Oehninger S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril. 2000; 74:1200–1207.
Article53. Badouard C, Ménézo Y, Panteix G, Ravanat JL, Douki T, Cadet J, et al. Determination of new types of DNA lesions in human sperm. Zygote. 2008; 16:9–13.
Article54. González-Marín C, Gosálvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 2012; 13:14026–14052.
Article55. Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2'-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009; 27:120–139.56. Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011; 13:36–42.
Article57. Aitken RJ, Baker MA. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int J Dev Biol. 2013; 57:265–272.
Article58. Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003; 9:331–345.
Article59. Kao SH, Chao HT, Chen HW, Hwang TI, Liao TL, Wei YH. Increase of oxidative stress in human sperm with lower motility. Fertil Steril. 2008; 89:1183–1190.
Article60. du Plessis SS, McAllister DA, Luu A, Savia J, Agarwal A, Lampiao F. Effects of H(2)O(2) exposure on human sperm motility parameters, reactive oxygen species levels and nitric oxide levels. Andrologia. 2010; 42:206–210.
Article61. Koppers AJ, Mitchell LA, Wang P, Lin M, Aitken RJ. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J. 2011; 436:687–698.
Article62. Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, Baker MA. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem. 2012; 287:33048–33060.
Article63. La Vignera S, Condorelli RA, Vicari E, D'Agata R, Calogero AE. Effects of the exposure to mobile phones on male reproduction: a review of the literature. J Androl. 2012; 33:350–356.
Article64. Miraglia E, De Angelis F, Gazzano E, Hassanpour H, Bertagna A, Aldieri E, et al. Nitric oxide stimulates human sperm motility via activation of the cyclic GMP/protein kinase G signaling pathway. Reproduction. 2011; 141:47–54.
Article65. Doshi SB, Khullar K, Sharma RK, Agarwal A. Role of reactive nitrogen species in male infertility. Reprod Biol Endocrinol. 2012; 10:109.
Article66. Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol. 2008; 59:2–11.67. Kefer JC, Agarwal A, Sabanegh E. Role of antioxidants in the treatment of male infertility. Int J Urol. 2009; 16:449–457.
Article68. Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril. 2006; 86:503–512.
Article69. Lampiao F. Free radicals generation in an in vitro fertilization setting and how to minimize them. World J Obstet Gynecol. 2012; 1:29–34.70. du Plessis SS, Makker K, Desai NR, Agarwal A. Impact of oxidative stress on IVF. Obstet Gynecol. 2008; 34:539–554.
Article71. Deepinder F, Cocuzza M, Agarwal A. Should seminal oxidative stress measurement be offered routinely to men presenting for infertility evaluation? Endocr Pract. 2008; 14:484–491.
Article72. Aydemir B, Onaran I, Kiziler AR, Alici B, Akyolcu MC. The influence of oxidative damage on viscosity of seminal fluid in infertile men. J Androl. 2008; 29:41–46.
Article73. Wang Y, Liang CL, Wu JQ, Xu C, Qin SX, Gao ES. Do Ureaplasma urealyticum infections in the genital tract affect semen quality? Asian J Androl. 2006; 8:562–568.
Article74. Zorn B, Sesek-Briski A, Osredkar J, Meden-Vrtovec H. Semen polymorphonuclear neutrophil leukocyte elastase as a diagnostic and prognostic marker of genital tract inflammation--a review. Clin Chem Lab Med. 2003; 41:2–12.
Article75. Menkveld R. Clinical significance of the low normal sperm morphology value as proposed in the fifth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Asian J Androl. 2010; 12:47–58.
Article76. Aitken RJ, Baker MA, O'Bryan M. Shedding light on chemiluminescence: the application of chemiluminescence in diagnostic andrology. J Androl. 2004; 25:455–465.77. Benjamin D, Sharma RK, Moazzam A, Agarwal A. Methods for the detection of ROS in human sperm samples. In : Agarwal A, Aitken RJ, Alvarez JG, editors. Studies on Men's Health and Fertility. New York: Humana Press;2012. p. 257–274.78. Jamsai D, O'Bryan MK. Genome-wide ENU mutagenesis for the discovery of novel male fertility regulators. Syst Biol Reprod Med. 2010; 56:246–259.
Article79. Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996; 48:835–850.
Article80. Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004; 8:616–627.
Article81. Zini A, Al-Hathal N. Antioxidant therapy in male infertility: fact or fiction? Asian J Androl. 2011; 13:374–381.
Article82. Mora-Esteves C, Shin D. Nutrient supplementation: improving male fertility fourfold. Semin Reprod Med. 2013; 31:293–300.
Article83. Griveau JF, Le Lannou D. Effects of antioxidants on human sperm preparation techniques. Int J Androl. 1994; 17:225–231.84. Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993; 215:213–219.
Article85. Lombardo F, Sansone A, Romanelli F, Paoli D, Gandini L, Lenzi A. The role of antioxidant therapy in the treatment of male infertility: an overview. Asian J Androl. 2011; 13:690–697.
Article86. Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012; 9:678–690.
Article87. Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013; 10:26–37.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Antioxidants in Assisted Reproductive Techniques
- The Role of Endoplasmic Reticulum Stress Response in Male Reproductive Physiology and Pathology: A Review
- Oxidative stress and endometriosis
- Epilepsy and Oxidative Stress
- Therapeutic Efficacy of Methanol Extract of Bidens tripartita in HT22 Cells by Neuroprotective Effect