World J Mens Health.
2015 Apr;33(1):20-29. 10.5534/wjmh.2015.33.1.20.
Protective Effect of Administered Rolipram against Radiation-Induced Testicular Injury in Mice
- Affiliations
-
- 1Department of Urology, Dongnam Institute of Radiological and Medical Sciences, Busan, Korea.
- 2Research Center, Dongnam Institute of Radiological and Medical Sciences, Busan, Korea. mildream@hanmail.net
- 3Medstar Washington Hospital Center, Georgetown University School of Medicine, Washington, DC, USA.
- 4Graduate School of Bio-Applications and System Engineering, Tokyo University of Agriculture and Technology, Tokyo, Japan.
- 5Department of Urology, Inje University College of Medicine, Busan, Korea.
- 6College of Veterinary Medicine, Chonnam National University, Gwangju, Korea.
- KMID: 2320736
- DOI: http://doi.org/10.5534/wjmh.2015.33.1.20
Abstract
- PURPOSE
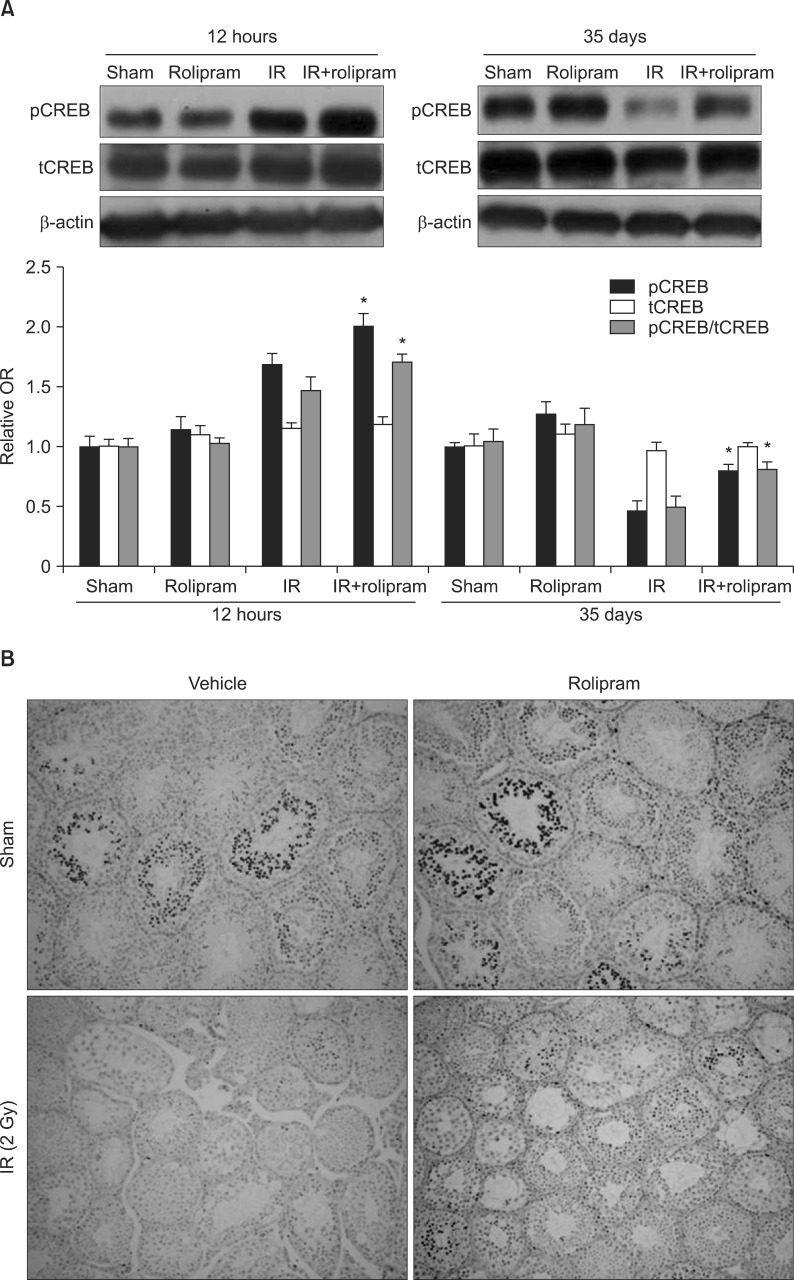
Pelvic irradiation for the treatment of cancer can affect normal cells, such as the rapidly proliferating spermatogenic cells of the testis, leading to infertility, a common post-irradiation problem. The present study investigated the radioprotective effect of rolipram, a specific phosphodiesterase type-IV inhibitor known to increase the expression and phosphorylation of the cyclic adenosine monophosphate response element-binding protein (CREB), a key factor for spermatogenesis, with the testicular system against pelvic irradiation.
MATERIALS AND METHODS
Male C57BL/6 mice were treated with pelvic irradiation (2 Gy) and rolipram, alone or in combination, and were sacrificed at 12 hours and 35 days after irradiation.
RESULTS
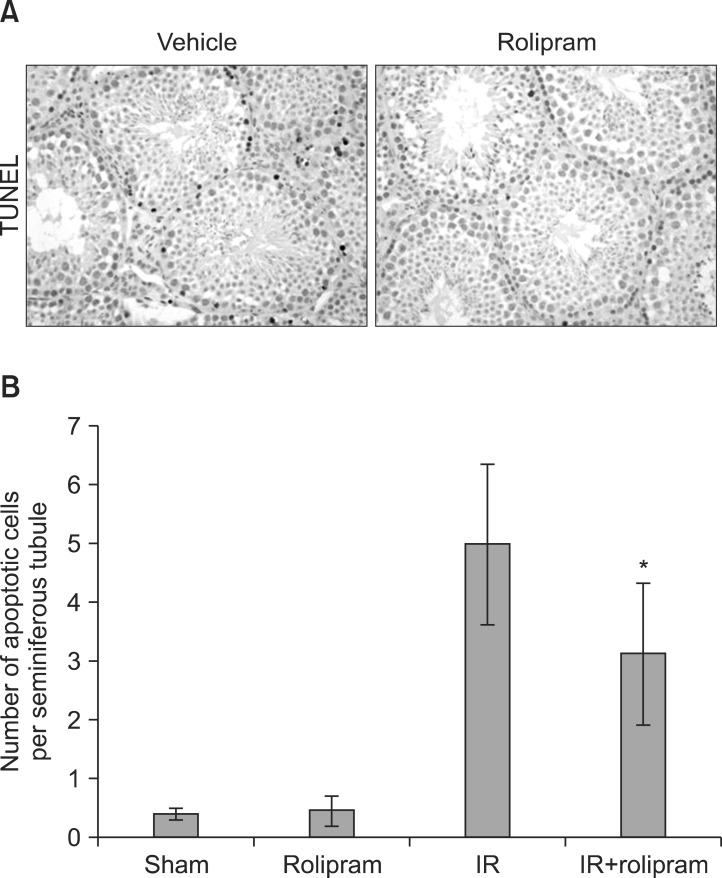
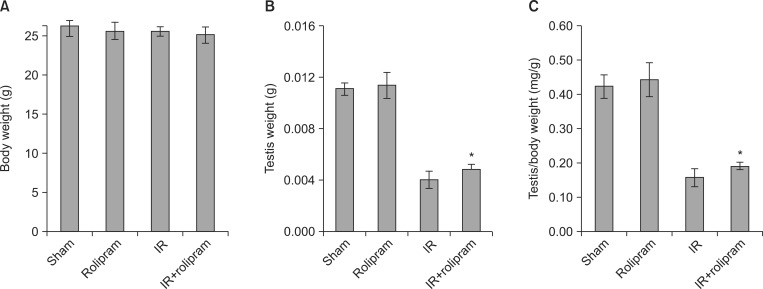
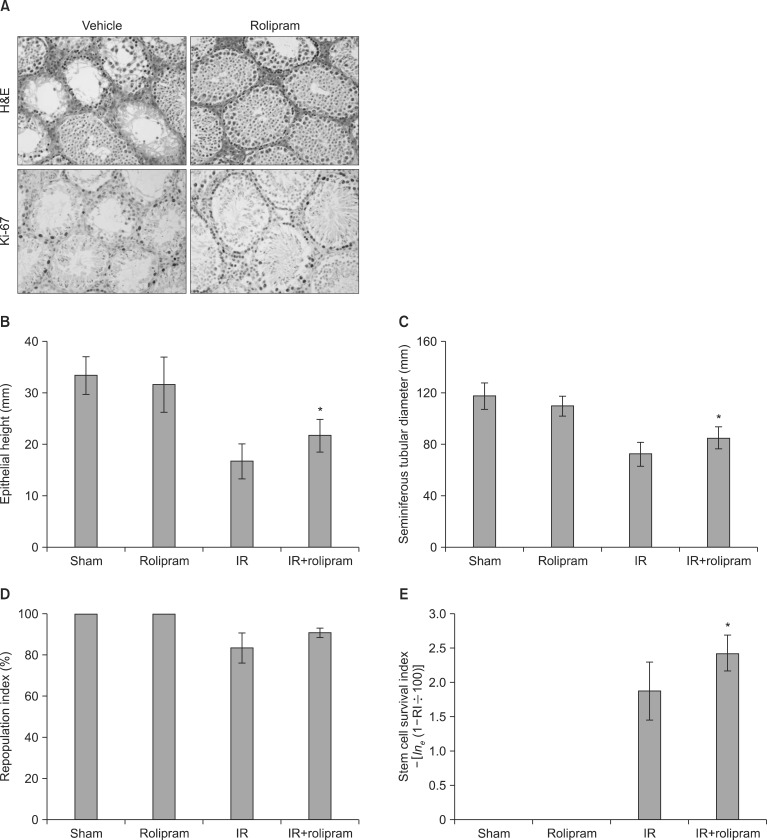
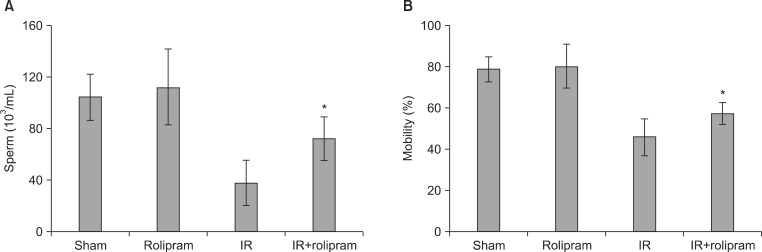
Rolipram protected germ cells from radiation-induced apoptosis at 12 hours after irradiation and significantly increased testis weight compared with irradiation controls at 35 days. Rolipram also ameliorated radiation-induced testicular morphological changes, such as changes in seminiferous tubular diameter and epithelial height. Additionally, seminiferous tubule repopulation and stem cell survival indices were higher in the rolipram-treated group than in the radiation group. Moreover, rolipram treatment counteracted the radiation-mediated decrease in the sperm count and mobility in the epididymis.
CONCLUSIONS
These protective effects of rolipram treatment prior to irradiation may be mediated by the increase in pCREB levels at 12 hours post-irradiation and the attenuated decrease in pCREB levels in the testis at 35 days post-irradiation in the rolipram-treated group. These findings suggest that activation of CREB signaling by rolipram treatment ameliorates the detrimental effects of acute irradiation on testicular dysfunction and the related male reproductive functions in mice.
MeSH Terms
Figure
Reference
-
1. Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 2005; (34):12–17. PMID: 15784814.
Article2. Budgell GJ, Cowan RA, Hounsell AR. Prediction of scattered dose to the testes in abdominopelvic radiotherapy. Clin Oncol (R Coll Radiol). 2001; 13:120–125. PMID: 11373874.
Article3. Kovacs GT, Stern K. Reproductive aspects of cancer treatment: an update. Med J Aust. 1999; 170:495–497. PMID: 10376028.
Article4. Costabile RA. The effects of cancer and cancer therapy on male reproductive function. J Urol. 1993; 149:1327–1330. PMID: 8479029.
Article5. Montminy M. Transcriptional activation. Something new to hang your HAT on. Nature. 1997; 387:654–655. PMID: 9192883.6. Don J, Stelzer G. The expanding family of CREB/CREM transcription factors that are involved with spermatogenesis. Mol Cell Endocrinol. 2002; 187:115–124. PMID: 11988318.
Article7. Kim JS, Song MS, Seo HS, Yang M, Kim SH, Kim JC, et al. Immunohistochemical analysis of cAMP response element-binding protein in mouse testis during postnatal development and spermatogenesis. Histochem Cell Biol. 2009; 131:501–507. PMID: 19148668.
Article8. Nagakura A, Niimura M, Takeo S. Effects of a phosphodiesterase IV inhibitor rolipram on microsphere embolism-induced defects in memory function and cerebral cyclic AMP signal transduction system in rats. Br J Pharmacol. 2002; 135:1783–1793. PMID: 11934820.
Article9. Vitolo OV, Sant'Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002; 99:13217–13221. PMID: 12244210.10. Kim JS, Yang M, Cho J, Kim SH, Kim JC, Shin T, et al. Promotion of cAMP responsive element-binding protein activity ameliorates radiation-induced suppression of hippocampal neurogenesis in adult mice. Toxicol Res. 2010; 26:177–183. PMID: 24278522.
Article11. Li YF, Huang Y, Amsdell SL, Xiao L, O'Donnell JM, Zhang HT. Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology. 2009; 34:2404–2419. PMID: 19516250.
Article12. Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004; 114:1624–1634. PMID: 15578094.
Article13. Monti B, Berteotti C, Contestabile A. Subchronic rolipram delivery activates hippocampal CREB and arc, enhances retention and slows down extinction of conditioned fear. Neuropsychopharmacology. 2006; 31:278–286. PMID: 15988467.
Article14. Wang C, Yang XM, Zhuo YY, Zhou H, Lin HB, Cheng YF, et al. The phosphodiesterase-4 inhibitor rolipram reverses A β-induced cognitive impairment and neuroinflammatory and apoptotic responses in rats. Int J Neuropsychopharmacol. 2012; 15:749–766. PMID: 21733236.15. Mammadov E, Aridogan IA, Izol V, Acikalin A, Abat D, Tuli A, et al. Protective effects of phosphodiesterase-4-specific inhibitor rolipram on acute ischemia-reperfusion injury in rat kidney. Urology. 2012; 80:1390.e1. 1390.e6. PMID: 23010343.
Article16. Parkkonen J, Hasala H, Moilanen E, Giembycz MA, Kankaanranta H. Phosphodiesterase 4 inhibitors delay human eosinophil and neutrophil apoptosis in the absence and presence of salbutamol. Pulm Pharmacol Ther. 2008; 21:499–506. PMID: 18282775.
Article17. Kim J, Lee S, Jeon B, Jang W, Moon C, Kim S. Protection of spermatogenesis against gamma ray-induced damage by granulocyte colony-stimulating factor in mice. Andrologia. 2011; 43:87–93. PMID: 21382061.
Article18. Kim JS, Heo K, Yi JM, Gong EJ, Yang K, Moon C, et al. Genistein mitigates radiation-induced testicular injury. Phytother Res. 2012; 26:1119–1125. PMID: 22162311.
Article19. Pugazhenthi S, Miller E, Sable C, Young P, Heidenreich KA, Boxer LM, et al. Insulin-like growth factor-I induces bcl-2 promoter through the transcription factor cAMP-response element-binding protein. J Biol Chem. 1999; 274:27529–27535. PMID: 10488088.
Article20. Walton M, Woodgate AM, Muravlev A, Xu R, During MJ, Dragunow M. CREB phosphorylation promotes nerve cell survival. J Neurochem. 1999; 73:1836–1842. PMID: 10537041.21. Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 2010; 185:6413–6419. PMID: 21084670.
Article22. François F, Godinho MJ, Grimes ML. CREB is cleaved by caspases during neural cell apoptosis. FEBS Lett. 2000; 486:281–284. PMID: 11119719.
Article23. Liu G, Gong P, Zhao H, Wang Z, Gong S, Cai L. Effect of low-level radiation on the death of male germ cells. Radiat Res. 2006; 165:379–389. PMID: 16579650.
Article24. Lee HJ, Kim JS, Moon C, Kim JC, Jo SK, Kim SH. Relative biological effectiveness of fast neutrons in a multiorgan assay for apoptosis in mouse. Environ Toxicol. 2008; 23:233–239. PMID: 18214905.
Article25. Cataldi A, di Giacomo V, Rapino M, Genovesi D, Rana RA. Cyclic nucleotide Response Element Binding protein (CREB) activation promotes survival signal in human K562 erythroleukemia cells exposed to ionising radiation/etoposide combined treatment. J Radiat Res. 2006; 47:113–120. PMID: 16819137.
Article26. Zhang H, Zheng RL, Wei ZQ, Li WJ, Gao QX, Chen WQ, et al. Effects of pre-exposure of mouse testis with low-dose (16)O8+ ions or 60Co gamma-rays on sperm shape abnormalities, lipid peroxidation and superoxide dismutase (SOD) activity induced by subsequent high-dose irradiation. Int J Radiat Biol. 1998; 73:163–167. PMID: 9489563.27. Kagan AR. Bladder, testicle, and prostate irradiation injury. Front Radiat Ther Oncol. 1989; 23:323–337. discussion 338-40. PMID: 2697661.
Article28. Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000; 182:311–322. PMID: 10653597.
Article29. Kim JS, Jung J, Lee HJ, Kim JC, Wang H, Kim SH, et al. Differences in immunoreactivities of Ki-67 and doublecortin in the adult hippocampus in three strains of mice. Acta Histochem. 2009; 111:150–156. PMID: 18649926.
Article30. Steger K, Aleithe I, Behre H, Bergmann M. The proliferation of spermatogonia in normal and pathological human seminiferous epithelium: an immunohistochemical study using monoclonal antibodies against Ki-67 protein and proliferating cell nuclear antigen. Mol Hum Reprod. 1998; 4:227–233. PMID: 9570268.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Phosphodiesterase-4-Specific Inhibitor in the Rat Model of Spinal Nerve Ligation
- Protective Effect of Several Metals Against Cadmium Injury to Mouse Testicle
- Radioprotective Effect of Mesna on Mouse Testis
- Effect of quercetin on impaired immune function in mice exposed to irradiation
- Rolipram, a Phosphodiesterase 4 Inhibitor, Stimulates Inducible cAMP Early Repressor Expression in Osteoblasts