Restor Dent Endod.
2015 Nov;40(4):290-298. 10.5395/rde.2015.40.4.290.
Effect of dentin treatment on proliferation and differentiation of human dental pulp stem cells
- Affiliations
-
- 1Department of Conservative Dentistry, Yonsei University College of Dentistry, Seoul, Korea. juen@yuhs.ac
- 2Department of Advanced General Dentistry, Oral Science Research Center, Yonsei University College of Dentistry, Seoul, Korea.
- KMID: 2316949
- DOI: http://doi.org/10.5395/rde.2015.40.4.290
Abstract
OBJECTIVES
Sodium hypochlorite (NaOCl) is an excellent bactericidal agent, but it is detrimental to stem cell survival, whereas intracanal medicaments such as calcium hydroxide (Ca[OH]2) promote the survival and proliferation of stem cells. This study evaluated the effect of sequential NaOCl and Ca[OH]2 application on the attachment and differentiation of dental pulp stem cells (DPSCs).
MATERIALS AND METHODS
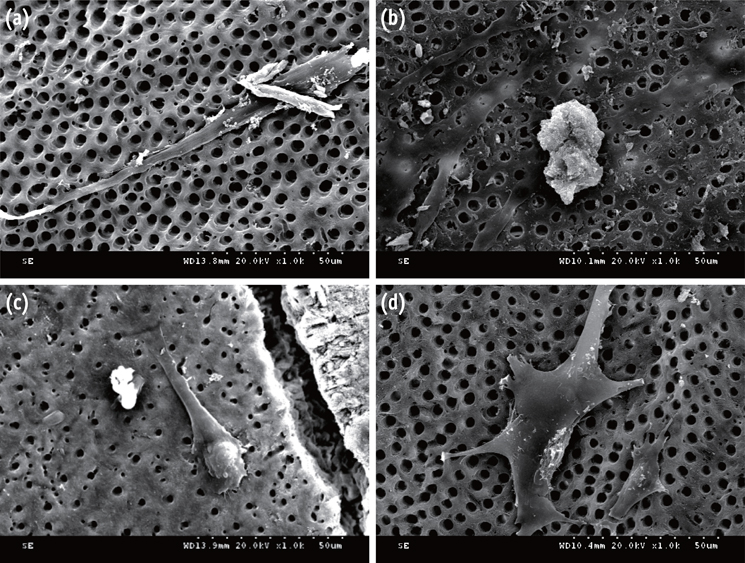
DPSCs were obtained from human third molars. All dentin specimens were treated with 5.25% NaOCl for 30 min. DPSCs were seeded on the dentin specimens and processed with additional 1 mg/mL Ca[OH]2, 17% ethylenediaminetetraacetic acid (EDTA) treatment, file instrumentation, or a combination of these methods. After 7 day of culture, we examined DPSC morphology using scanning electron microscopy and determined the cell survival rate with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. We measured cell adhesion gene expression levels after 4 day of culture and odontogenic differentiation gene expression levels after 4 wk using quantitative real-time polymerase chain reaction.
RESULTS
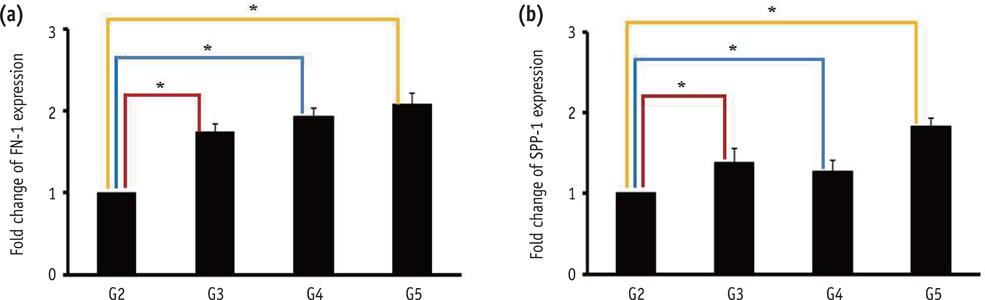
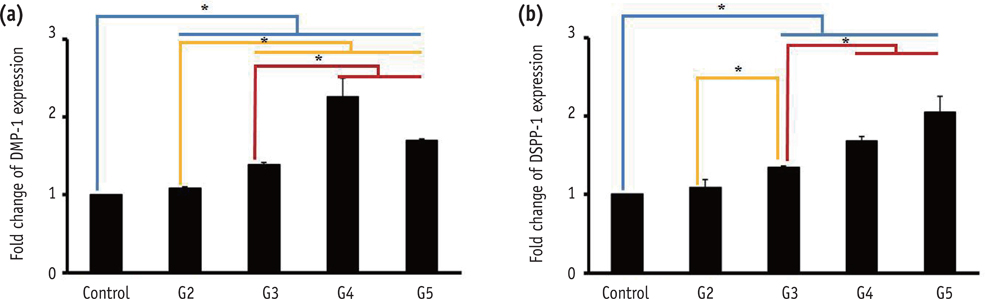
DPSCs did not attach to the dentin in the NaOCl-treated group. The gene expression levels of fibronectin-1 and secreted phosphoprotein-1 gene in both the Ca[OH]2- and the EDTA-treated groups were significantly higher than those in the other groups. All Ca[OH]2-treated groups showed higher expression levels of dentin matrix protein-1 than that of the control. The dentin sialophosphoprotein level was significantly higher in the groups treated with both Ca[OH]2 and EDTA.
CONCLUSIONS
The application of Ca[OH]2 and additional treatment such as EDTA or instrumentation promoted the attachment and differentiation of DPSCs after NaOCl treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Jung IY, Lee SJ, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod. 2008; 34:876–887.
Article2. Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009; 35:1343–1349.
Article3. Ding RY, Cheung GS, Chen J, Yin XZ, Wang QQ, Zhang CF. Pulp revascularization of immature teeth with apical periodontitis: a clinical study. J Endod. 2009; 35:745–749.
Article4. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004; 30:196–200.
Article5. Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of the literature. Pediatr Dent. 2007; 29:47–50.6. Martin G, Ricucci D, Gibbs JL, Lin LM. Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J Endod. 2013; 39:138–144.
Article7. Trevino EG, Patwardhan AN, Henry MA, Perry G, Dybdal-Hargreaves N, Hargreaves KM, Diogenes A. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod. 2011; 37:1109–1115.
Article8. Wennberg A. Biological evaluation of root canal antiseptics using in vitro and in vivo methods. Scand J Dent Res. 1980; 88:46–52.
Article9. Chang YC, Huang FM, Tai KW, Chou MY. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001; 92:446–450.
Article10. Heling I, Rotstein I, Dinur T, Szwec-Levine Y, Steinberg D. Bactericidal and cytotoxic effects of sodium hypochlorite and sodium dichloroisocyanurate solutions in vitro. J Endod. 2001; 27:278–280.
Article11. Ring KC, Murray PE, Namerow KN, Kuttler S, Garcia-Godoy F. The comparison of the effect of endodontic irrigation on cell adherence to root canal dentin. J Endod. 2008; 34:1474–1479.
Article12. Shah N, Logani A, Bhaskar U, Aggarwal V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: a pilot clinical study. J Endod. 2008; 34:919–925. Discussion 1157
Article13. Hoshino E, Kurihara-Ando N, Sato I, Uematsu H, Sato M, Kota K, Iwaku M. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996; 29:125–130.
Article14. Chueh LH, Ho YC, Kuo TC, Lai WH, Chen YH, Chiang CP. Regenerative endodontic treatment for necrotic immature permanent teeth. J Endod. 2009; 35:160–164.
Article15. Althumairy RI, Teixeira FB, Diogenes A. Effect of dentin conditioning with intracanal medicaments on survival of stem cells of apical papilla. J Endod. 2014; 40:521–525.
Article16. Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012; 38:1372–1375.
Article17. Pang NS, Lee SJ, Kim E, Shin DM, Cho SW, Park W, Zhang X, Jung IY. Effect of EDTA on attachment and differentiation of dental pulp stem cells. J Endod. 2014; 40:811–817.
Article18. Brännström M. Smear layer: pathological and treatment considerations. Oper Dent Suppl. 1984; 3:35–42.19. Czonstkowsky M, Wilson EG, Holstein FA. The smear layer in endodontics. Dent Clin North Am. 1990; 34:13–25.20. Takeda FH, Harashima T, Kimura Y, Matsumoto K. A comparative study of the removal of smear layer by three endodontic irrigants and two types of laser. Int Endod J. 1999; 32:32–39.
Article21. Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 94:658–666.
Article22. Guven EP, Yalvac ME, Sahin F, Yazici MM, Rizvanov AA, Bayirli G. Effect of dental materials calcium hydroxide-containing cement, mineral trioxide aggregate, and enamel matrix derivative on proliferation and differentiation of human tooth germ stem cells. J Endod. 2011; 37:650–656.
Article23. Kapila YL, Lancero H, Johnson PW. The response of periodontal ligament cells to fibronectin. J Periodontol. 1998; 69:1008–1019.
Article24. Noda M, Vogel RL, Craig AM, Prahl J, DeLuca HF, Denhardt DT. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 receptor and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl Acad Sci U S A. 1990; 87:9995–9999.
Article25. Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007; 6:997–1003.
Article26. Hayman EG, Pierschbacher MD, Suzuki S, Ruoslahti E. Vitronectin-a major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res. 1985; 160:245–258.
Article27. Calt S, Serper A. Dentinal tubule penetration of root canal sealers after root canal dressing with calcium hydroxide. J Endod. 1999; 25:431–433.
Article28. Kenee DM, Allemang JD, Johnson JD, Hellstein J, Nichol BK. A quantitative assessment of efficacy of various calcium hydroxide removal techniques. J Endod. 2006; 32:563–565.
Article29. Yue J, Wu B, Gao J, Huang X, Li C, Ma D, Fang F. DMP1 is a target of let-7 in dental pulp cells. Int J Mol Med. 2012; 30:295–301.
Article30. Martini D, Trirè A, Breschi L, Mazzoni A, Teti G, Falconi M, Ruggeri A Jr. Dentin matrix protein 1 and dentin sialophosphoprotein in human sound and carious teeth: an immunohistochemical and colorimetric assay. Eur J Histochem. 2013; 57:e32.
Article31. Graham L, Cooper PR, Cassidy N, Nor JE, Sloan AJ, Smith AJ. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials. 2006; 27:2865–2873.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem cell-derived exosomes for dentin-pulp complex regeneration: a mini-review
- Dlx3 and Dlx5 Inhibit Adipogenic Differentiation of Human Dental Pulp Stem Cells
- Chios gum mastic enhance the proliferation and odontogenic differentiation of human dental pulp stem cells
- Establishing Three-Dimensional Explant Culture of Human Dental Pulp Tissue
- Dental Pulp Stem Cells and Current in vivo Approaches to Study Dental Pulp Stem Cells in Pulp Injury and Regeneration