Korean J Physiol Pharmacol.
2024 Sep;28(5):423-433. 10.4196/kjpp.2024.28.5.423.
Chios gum mastic enhance the proliferation and odontogenic differentiation of human dental pulp stem cells
- Affiliations
-
- 1Department of Oral Anatomy, School of Dentistry, Pusan National University, Yangsan 50612, Korea
- 2Department of Pediatric Dentistry, School of Dentistry, Pusan National University, Yangsan 50612, Korea
- 3Department of Orthodontics, School of Dentistry, Pusan National University, Yangsan 50612, Korea
- 4Dental and Life Science Institute, School of Dentistry, Pusan National University, Yangsan 50612, Korea
- KMID: 2559135
- DOI: http://doi.org/10.4196/kjpp.2024.28.5.423
Abstract
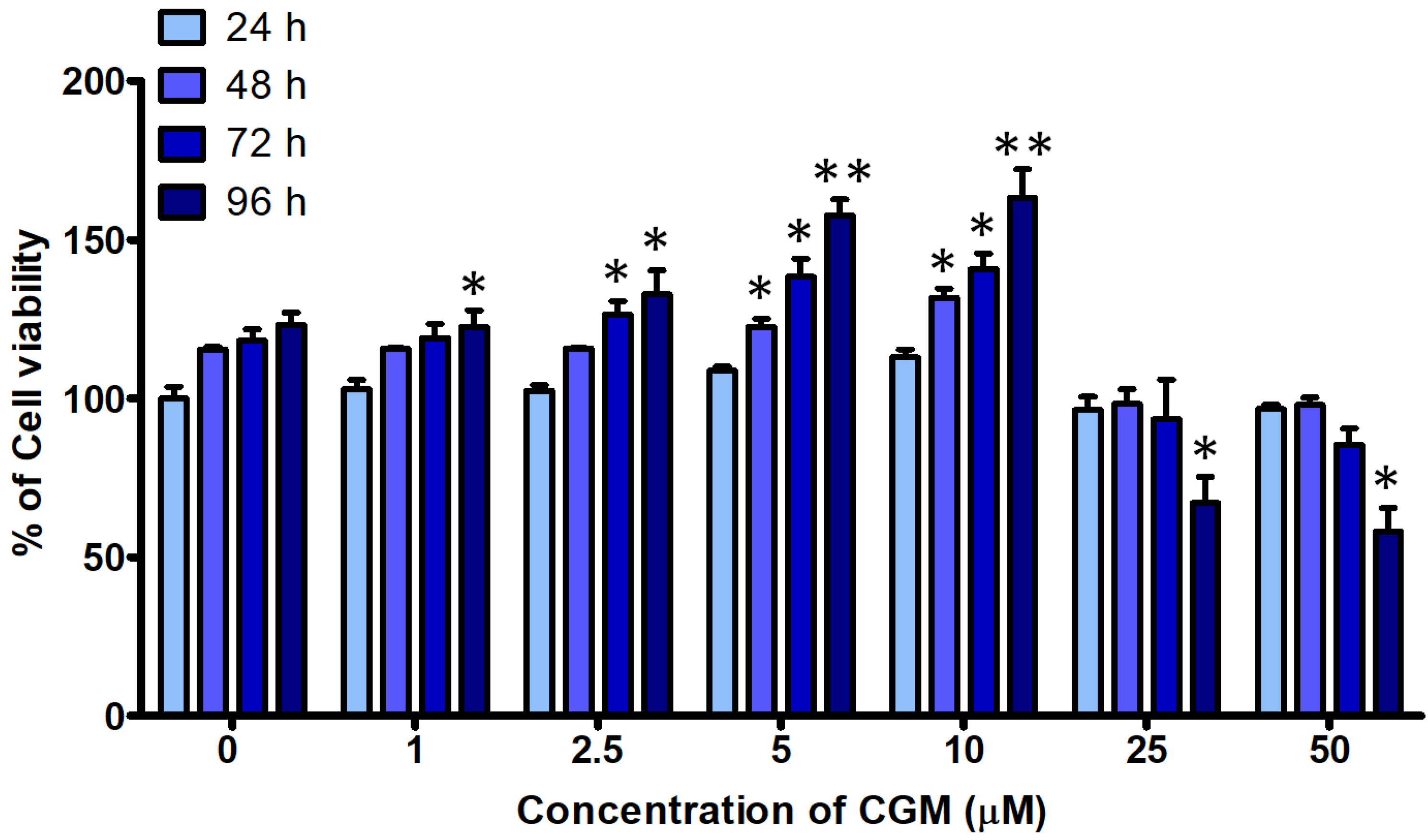
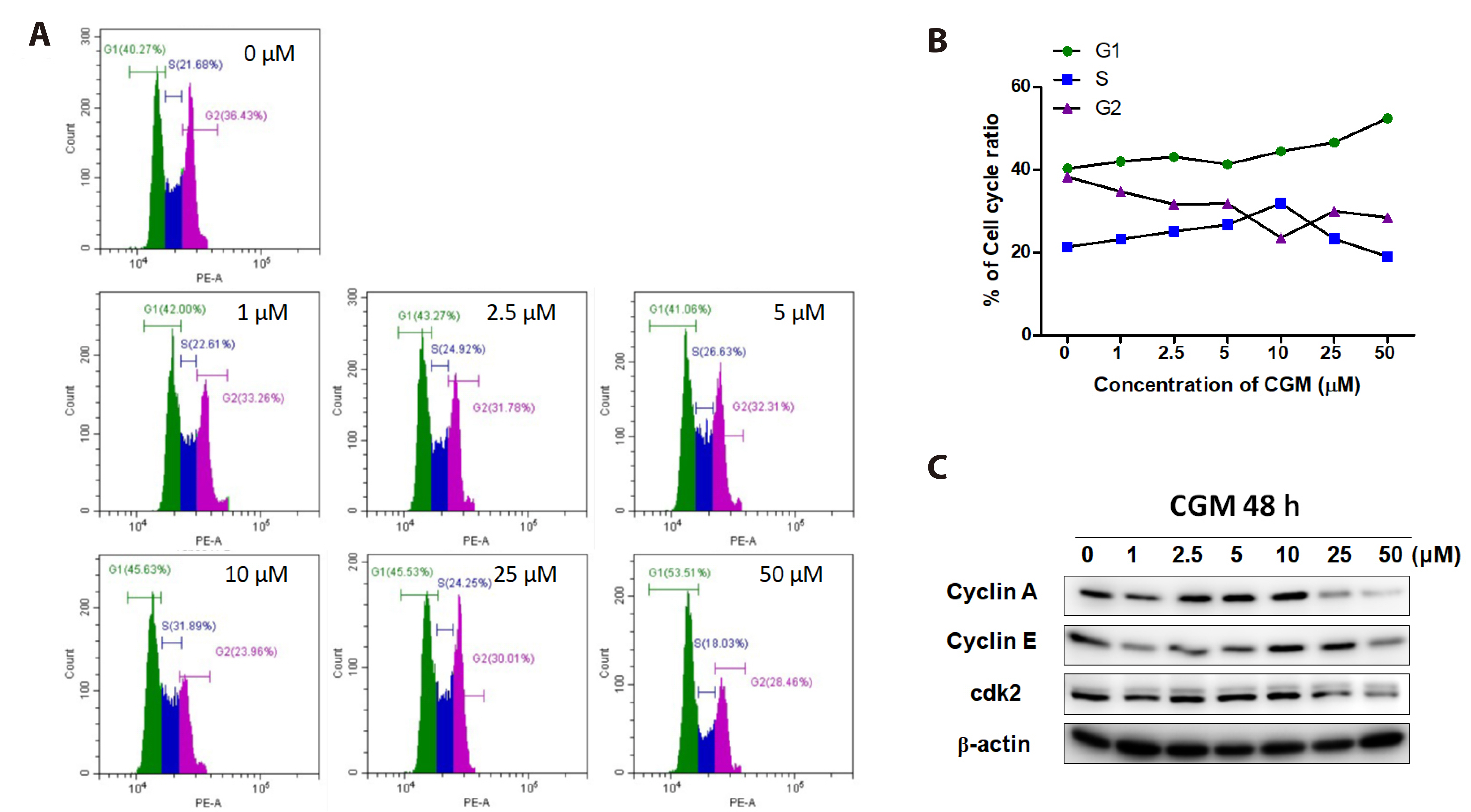
- Dental pulp stem cells (DPSCs) are a type of adult stem cell present in the dental pulp tissue. They possess a higher proliferative capacity than bone marrow mesenchymal stem cells. Their ease of collection from patients makes them well-suited for tissue engineering applications, such as tooth and nerve regeneration. Chios gum mastic (CGM), a resin extracted from the stems and leaves of Pistacia lentiscus var. Chia, has garnered attention for its potential in tissue regeneration. This study aims to confirm alterations in cell proliferation rates and induce differentiation in human DPSCs (hDPSCs) through CGM treatment, a substance known for effectively promoting odontogenic differentiation. Administration of CGM to hDPSC cells was followed by an assessment of cell survival, proliferation, and odontogenic differentiation through protein and gene analysis. The study revealed that hDPSCs exhibited low sensitivity to CGM toxicity. CGM treatment induced cell proliferation by activating cell-cycle proteins through the Wnt/β-catenin pathway. Additionally, the study demonstrated that CGM enhances alkaline phosphatase activation by upregulating the expression of collagen type I, a representative matrix protein of dentin. This activation of markers associated with odontogenic and bone differentiation ultimately facilitated the mineralization of hDPSCs. This study concludes that CGM, as a natural substance, fosters the cell cycle and cell proliferation in hDPSCs. Furthermore, it triggers the transcription of odontogenic and osteogenic markers, thereby facilitating odontogenic differentiation.
Figure
Reference
-
1. Kawashima N, Okiji T. 2016; Odontoblasts: specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit Anom (Kyoto). 56:144–153. DOI: 10.1111/cga.12169. PMID: 27131345.2. Parinyaprom N, Nirunsittirat A, Chuveera P, Na Lampang S, isuwan T Sr, Sastraruji T, Bua-On P, Simprasert S, Khoipanich I, Sutharaphan T, Theppimarn S, Ue-ichai N Sr, Tangtrakooljaroen W, Chompu-Inwai P. 2018; Outcomes of direct pulp capping by using either ProRoot mineral trioxide aggregate or biodentine in permanent teeth with carious pulp exposure in 6- to 18-year-old patients: a randomized controlled trial. J Endod. 44:341–348. DOI: 10.1016/j.joen.2017.10.012. PMID: 29275850.3. Nguyen TD. 2014. Mineral trioxide aggregate/ferric sulfate pulpotomy for vital primary incisors: a randomized controlled trial. [Master thesis]. University of Toronto;Toronto:4. Kim SG, Malek M, Sigurdsson A, Lin LM, Kahler B. 2018; Regenerative endodontics: a comprehensive review. Int Endod J. 51:1367–1388. DOI: 10.1111/iej.12954. PMID: 29777616.5. Suzuki T, Lee CH, Chen M, Zhao W, Fu SY, Qi JJ, Chotkowski G, Eisig SB, Wong A, Mao JJ. 2011; Induced migration of dental pulp stem cells for in vivo pulp regeneration. J Dent Res. 90:1013–1018. DOI: 10.1177/0022034511408426. PMID: 21586666.6. Kim SG, Zheng Y, Zhou J, Chen M, Embree MC, Song K, Jiang N, Mao JJ. 2013; Dentin and dental pulp regeneration by the patient's endogenous cells. Endod Topics. 28:106–117. DOI: 10.1111/etp.12037. PMID: 24976816. PMCID: PMC4070522.7. Huang X, Chen X, Chen H, Xu D, Lin C, Peng B. 2018; Rho/Rho-associated protein kinase signaling pathway-mediated downregulation of runt-related transcription factor 2 expression promotes the differentiation of dental pulp stem cells into odontoblasts. Exp Ther Med. 15:4457–4464. DOI: 10.3892/etm.2018.5982.8. Nuti N, Corallo C, Chan BM, Ferrari M, Gerami-Naini B. 2016; Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev Rep. 12:511–523. DOI: 10.1007/s12015-016-9661-9. PMID: 27240827.9. Tsutsui TW. 2020; Dental pulp stem cells: advances to applications. Stem Cells Cloning. 13:33–42. DOI: 10.2147/SCCAA.S166759. PMID: 32104005. PMCID: PMC7025818.10. Paino F, La Noce M, Giuliani A, De Rosa A, Mazzoni S, Laino L, Amler E, Papaccio G, Desiderio V, Tirino V. 2017; Human DPSCs fabricate vascularized woven bone tissue: a new tool in bone tissue engineering. Clin Sci (Lond). 131:699–713. DOI: 10.1042/CS20170047. PMID: 28209631. PMCID: PMC5383003.11. Georgiadis I, Karatzas T, Korou LM, Katsilambros N, Perrea D. 2015; Beneficial health effects of Chios Gum Mastic and peroxisome proliferator-activated receptors: indications of common mechanisms. J Med Food. 18:1–10. DOI: 10.1089/jmf.2014.0021. PMID: 25133901.12. Rauf A, Patel S, Uddin G, Siddiqui BS, Ahmad B, Muhammad N, Mabkhot YN, Hadda TB. 2017; Phytochemical, ethnomedicinal uses and pharmacological profile of genus Pistacia. Biomed Pharmacother. 86:393–404. DOI: 10.1016/j.biopha.2016.12.017. PMID: 28012394.13. Paraschos S, Mitakou S, Skaltsounis AL. 2012; Chios gum mastic: a review of its biological activities. Curr Med Chem. 19:2292–2302. DOI: 10.2174/092986712800229014. PMID: 22414110.14. Bayat H, Shahabinejad H, Bayat M, Shirian S, Mohamadnia A, Alijani M, Godarzi A, Shojaei P, Shojaei S, Shevidi A, Bahrami N. 2019; Osteogenic differentiation of follicular stem cells on nano-Saghez scaffold containing BMP2. J Orthop Surg Res. 14:442. DOI: 10.1186/s13018-019-1507-0. PMID: 31842947. PMCID: PMC6916075.15. Lee KH, Kim YS, Yu SB, Kang HM, Kwak HH, Kim IR, Park BS. 2016; Synergistic effects of Chios gum mastic extract and low level laser therapy on osteoblast differentiation. Int J Oral Biol. 41:53–62. DOI: 10.11620/IJOB.2016.41.2.053.16. Song JM, Park BS, Shin SH, Kim IR. 2021; Low-level laser irradiation stimulates RANKL-induced osteoclastogenesis via the MAPK pathway in RAW 264.7 cells. Appl Sci. 11:5360. DOI: 10.3390/app11125360.17. Teo JL, Kahn M. 2010; The Wnt signaling pathway in cellular proliferation and differentiation: a tale of two coactivators. Adv Drug Deliv Rev. 62:1149–1155. DOI: 10.1016/j.addr.2010.09.012. PMID: 20920541.18. Pathak K, Das RJ. 2013; Herbal medicine-a rational approach in health care system. Int J Herb Med. 1:86–89.19. Shakya AK. 2016; Medicinal plants: future source of new drugs. Int J Herb Med. 4:59–64.20. Xue W, Yu J, Chen W. 2018; Plants and their bioactive constituents in mesenchymal stem cell-based periodontal regeneration: a novel prospective. Biomed Res Int. 2018:7571363. DOI: 10.1155/2018/7571363. PMID: 30175141. PMCID: PMC6098897.21. Ashtiani RE, Hadi A, Nouri F, Rahimi S, Badkoobeh A, Abbasi K, Alam M. 2022; The role of current herbal extracts in bone regeneration through dental implants: in vitro/in vivo/clinical studies. Arch Med Sci. 19:1653–1661.22. Kokoska L, Kloucek P, Leuner O, Novy P. 2019; Plant-derived products as antibacterial and antifungal agents in human health care. Curr Med Chem. 26:5501–5541. DOI: 10.2174/0929867325666180831144344. PMID: 30182844.23. Dimas KS, Pantazis P, Ramanujam R. 2012; Review: Chios mastic gum: a plant-produced resin exhibiting numerous diverse pharmaceutical and biomedical properties. In Vivo. 26:777–785.24. Davidson G, Niehrs C. 2010; Emerging links between CDK cell cycle regulators and Wnt signaling. Trends Cell Biol. 20:453–460. DOI: 10.1016/j.tcb.2010.05.002. PMID: 20627573.25. Niehrs C, Acebron SP. 2012; Mitotic and mitogenic Wnt signalling. EMBO J. 31:2705–2713. DOI: 10.1038/emboj.2012.124. PMID: 22617425. PMCID: PMC3380213.26. Malumbres M, Barbacid M. 2005; Mammalian cyclin-dependent kinases. Trends Biochem Sci. 30:630–641. DOI: 10.1016/j.tibs.2005.09.005. PMID: 16236519.27. Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, Zhou Z, Shu G, Yin G. 2022; Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 7:3. DOI: 10.1038/s41392-021-00762-6. PMID: 34980884. PMCID: PMC8724284.28. Aurrekoetxea M, Irastorza I, García-Gallastegui P, Jiménez-Rojo L, Nakamura T, Yamada Y, Ibarretxe G, Unda FJ. 2016; Wnt/β-catenin regulates the activity of Epiprofin/Sp6, SHH, FGF, and BMP to coordinate the stages of odontogenesis. Front Cell Dev Biol. 4:25. DOI: 10.3389/fcell.2016.00025. PMID: 27066482. PMCID: PMC4811915.29. Zhang H, Wang J, Deng F, Huang E, Yan Z, Wang Z, Deng Y, Zhang Q, Zhang Z, Ye J, Qiao M, Li R, Wang J, Wei Q, Zhou G, Luu HH, Haydon RC, He TC, Deng F. 2015; Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs). Biomaterials. 39:145–154. DOI: 10.1016/j.biomaterials.2014.11.007. PMID: 25468367. PMCID: PMC4258144.30. Huang P, Senga T, Hamaguchi M. 2007; A novel role of phospho-beta-catenin in microtubule regrowth at centrosome. Oncogene. 26:4357–4371. DOI: 10.1038/sj.onc.1210217. PMID: 17260019.31. Fumoto K, Kadono M, Izumi N, Kikuchi A. 2009; Axin localizes to the centrosome and is involved in microtubule nucleation. EMBO Rep. 10:606–613. DOI: 10.1038/embor.2009.45. PMID: 19390532. PMCID: PMC2711835.32. Hadjihannas MV, Brückner M, Behrens J. 2010; Conductin/axin2 and Wnt signalling regulates centrosome cohesion. EMBO Rep. 11:317–324. DOI: 10.1038/embor.2010.23. PMID: 20300119. PMCID: PMC2854593.33. Garzón I, Martin-Piedra MA, Carriel V, Alaminos M, Liu X, D'Souza RN. 2018; Bioactive injectable aggregates with nanofibrous microspheres and human dental pulp stem cells: a translational strategy in dental endodontics. J Tissue Eng Regen Med. 12:204–216. DOI: 10.1002/term.2397. PMID: 28079309.34. Golub EE, Boesze-Battaglia K. 2007; The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 18:444–448. DOI: 10.1097/BCO.0b013e3282630851.35. Sowmya S, Bumgardener JD, Chennazhi KP, Nair SV, Jayakumar R. 2013; Role of nanostructured biopolymers and bioceramics in enamel, dentin and periodontal tissue regeneration. Prog Polym Sci. 38:1748–1772. DOI: 10.1016/j.progpolymsci.2013.05.005.36. Xu C, Wang Y. 2012; Chemical composition and structure of peritubular and intertubular human dentine revisited. Arch Oral Biol. 57:383–391. DOI: 10.1016/j.archoralbio.2011.09.008. PMID: 21996490. PMCID: PMC3276734.37. Bertassoni LE, Orgel JP, Antipova O, Swain MV. 2012; The dentin organic matrix - limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater. 8:2419–2433. DOI: 10.1016/j.actbio.2012.02.022. PMID: 22414619. PMCID: PMC3473357.38. Butler WT, Ritchie HH, Bronckers AL. 1997; Extracellular matrix proteins of dentine. Ciba Found Symp. 205:107–115. discussion 115-117. DOI: 10.1002/9780470515303.ch8. PMID: 9189620.39. Goldberg M, Kulkarni AB, Young M, Boskey A. 2011; Dentin: structure, composition and mineralization. Front Biosci (Elite Ed). 3:711–735. DOI: 10.2741/e281. PMID: 21196346. PMCID: PMC3360947.40. Liu Y, Liu N, Na J, Li C, Yue G, Fan Y, Zheng L. 2023; Wnt/β-catenin plays a dual function in calcium hydroxide induced proliferation, migration, osteogenic differentiation and mineralization in vitro human dental pulp stem cells. Int Endod J. 56:92–102. DOI: 10.1111/iej.13843. PMID: 36229421.41. Bakopoulou A, Leyhausen G, Volk J, Papachristou E, Koidis P, Geurtsen W. 2015; Wnt/β-catenin signaling regulates Dental Pulp Stem Cells' responses to pulp injury by resinous monomers. Dent Mater. 31:542–555. DOI: 10.1016/j.dental.2015.02.004. PMID: 25735758.42. Scheller EL, Chang J, Wang CY. 2008; Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 87:126–130. DOI: 10.1177/154405910808700206. PMID: 18218837. PMCID: PMC2906770.43. Zhou Y, Lin J, Shao J, Zuo Q, Wang S, Wolff A, Nguyen DT, Rintoul L, Du Z, Gu Y, Peng YY, Ramshaw JAM, Long X, Xiao Y. 2019; Aberrant activation of Wnt signaling pathway altered osteocyte mineralization. Bone. 127:324–333. DOI: 10.1016/j.bone.2019.06.027. PMID: 31260814.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem cell-derived exosomes for dentin-pulp complex regeneration: a mini-review
- Dlx3 and Dlx5 Inhibit Adipogenic Differentiation of Human Dental Pulp Stem Cells
- The Inhibition of Oxidative Stress by Chios Gum Mastic is Associated with Autophagy
- Effects of CTHRC1 on odontogenic differentiation and angiogenesis in human dental pulp stem cells
- A Trial of Screening of Genes Involved in Odontoblasts Differentiation from Human Dental Pulp Stem Cells