Restor Dent Endod.
2023 May;48(2):e18. 10.5395/rde.2023.48.e18.
Effects of CTHRC1 on odontogenic differentiation and angiogenesis in human dental pulp stem cells
- Affiliations
-
- 1Department of Conservative Dentistry, School of Dentistry, Chonnam National University, Gwangju, Korea
- KMID: 2548221
- DOI: http://doi.org/10.5395/rde.2023.48.e18
Abstract
Objectives
This study aimed to determine whether collagen triple helix repeat containing-1 (CTHRC1), which is involved in vascular remodeling and bone formation, can stimulate odontogenic differentiation and angiogenesis when administered to human dental pulp stem cells (hDPSCs).
Materials and Methods
The viability of hDPSCs upon exposure to CTHRC1 was assessed with the WST-1 assay. CTHRC1 doses of 5, 10, and 20 µg/mL were administered to hDPSCs. Reverse-transcription polymerase reaction was used to detect dentin sialophosphoprotein, dentin matrix protein 1, vascular endothelial growth factor, and fibroblast growth factor 2. The formation of mineralization nodules was evaluated using Alizarin red. A scratch wound assay was conducted to evaluate the effect of CTHRC1 on cell migration. Data were analyzed using 1-way analysis of variance followed by the Tukey post hoc test. The threshold for statistical significance was set at p < 0.05.
Results
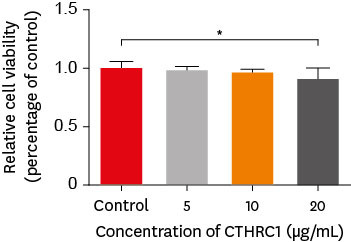
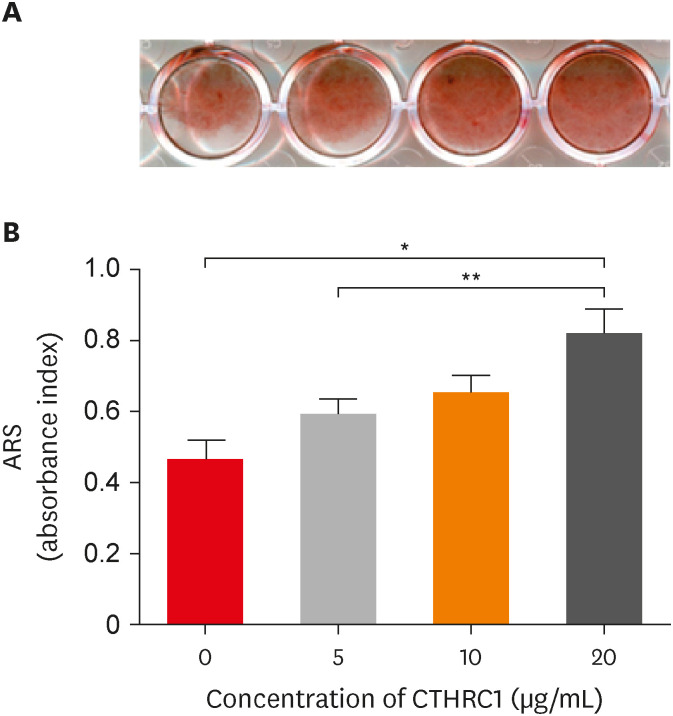
CTHRC1 doses of 5, 10, and 20 µg/mL had no significant effect on the viability of hDPSCs. Mineralized nodules were formed and odontogenic markers were upregulated, indicating that CTHRC1 promoted odontogenic differentiation. Scratch wound assays demonstrated that CTHRC1 significantly enhanced the migration of hDPSCs.
Conclusions
CTHRC1 promoted odontogenic differentiation and mineralization in hDPSCs.
Keyword
Figure
Reference
-
1. Nair PN. On the causes of persistent apical periodontitis: a review. Int Endod J. 2006; 39:249–281. PMID: 16584489.
Article2. Goto Y, Ceyhan J, Chu SJ. Restorations of endodontically treated teeth: new concepts, materials, and aesthetics. Pract Proced Aesthet Dent. 2009; 21:81–89. PMID: 19583165.3. Glickman GN, Koch KA. 21st-century endodontics. J Am Dent Assoc. 2000; 131(Suppl):39S–46S. PMID: 10860344.
Article4. Lin LM, Di Fiore PM, Lin J, Rosenberg PA. Histological study of periradicular tissue responses to uninfected and infected devitalized pulps in dogs. J Endod. 2006; 32:34–38. PMID: 16410065.
Article5. Rocha CT, Rossi MA, Leonardo MR, Rocha LB, Nelson-Filho P, Silva LA. Biofilm on the apical region of roots in primary teeth with vital and necrotic pulps with or without radiographically evident apical pathosis. Int Endod J. 2008; 41:664–669. PMID: 18479368.
Article6. Valderhaug J, Jokstad A, Ambjørnsen E, Norheim PW. Assessment of the periapical and clinical status of crowned teeth over 25 years. J Dent. 1997; 25:97–105. PMID: 9105139.
Article7. Maurice CG. American Association of Endodontists. An annotated glossary of terms used in endodontics. Oral Surg Oral Med Oral Pathol. 1968; 25:491–512. PMID: 5237485.
Article8. Baume LJ. The biology of pulp and dentine. A historic, terminologic-taxonomic, histologic-biochemical, embryonic and clinical survey. Monogr Oral Sci. 1980; 8:1–220. PMID: 6986016.9. Granath L. Pulp capping materials. Biocompat Dent Mater. 1982; 2:253–267.10. Schröder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res. 1985; 64:541–548. PMID: 3857254.
Article11. Stanley HR. Pulp capping: conserving the dental pulp--can it be done? Is it worth it? Oral Surg Oral Med Oral Pathol. 1989; 68:628–639. PMID: 2682429.
Article12. Berman MH. Pulpotomy: the old reliable pulp therapy. Dent Today. 1996; 15:60.13. Stanley HR. Criteria for standardizing and increasing credibility of direct pulp capping studies. Am J Dent. 1998; 11:S17–S34.14. Durmus T, LeClair RJ, Park KS, Terzic A, Yoon JK, Lindner V. Expression analysis of the novel gene collagen triple helix repeat containing-1 (Cthrc1). Gene Expr Patterns. 2006; 6:935–940. PMID: 16678498.
Article15. Pyagay P, Heroult M, Wang Q, Lehnert W, Belden J, Liaw L, Friesel RE, Lindner V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res. 2005; 96:261–268. PMID: 15618538.
Article16. Tang L, Dai DL, Su M, Martinka M, Li G, Zhou Y. Aberrant expression of collagen triple helix repeat containing 1 in human solid cancers. Clin Cancer Res. 2006; 12:3716–3722. PMID: 16778098.
Article17. Duarte CW, Stohn JP, Wang Q, Emery IF, Prueser A, Lindner V. Elevated plasma levels of the pituitary hormone Cthrc1 in individuals with red hair but not in patients with solid tumors. PLoS One. 2014; 9:e100449. PMID: 24945147.
Article18. Stohn JP, Perreault NG, Wang Q, Liaw L, Lindner V. Cthrc1, a novel circulating hormone regulating metabolism. PLoS One. 2012; 7:e47142. PMID: 23056600.
Article19. Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003; 60:107–114. PMID: 12500267.
Article20. Kimura H, Kwan KM, Zhang Z, Deng JM, Darnay BG, Behringer RR, Nakamura T, de Crombrugghe B, Akiyama H. Cthrc1 is a positive regulator of osteoblastic bone formation. PLoS One. 2008; 3:e3174. PMID: 18779865.
Article21. Takeshita S, Fumoto T, Matsuoka K, Park KA, Aburatani H, Kato S, Ito M, Ikeda K. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest. 2013; 123:3914–3924. PMID: 23908115.
Article22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001; 25:402–408. PMID: 11846609.
Article23. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo . Proc Natl Acad Sci U S A. 2000; 97:13625–13630. PMID: 11087820.24. Smith AJ, Lesot H. Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med. 2001; 12:425–437. PMID: 12002824.
Article25. Ding X, Huang R, Zhong Y, Cui N, Wang Y, Weng J, Chen L, Zang M. CTHRC1 promotes gastric cancer metastasis via HIF-1α/CXCR4 signaling pathway. Biomed Pharmacother. 2020; 123:109742. PMID: 31855733.
Article26. Li J, Wang Y, Ma M, Jiang S, Zhang X, Zhang Y, Yang X, Xu C, Tian G, Li Q, Wang Y, Zhu L, Nie H, Feng M, Xia Q, Gu J, Xu Q, Zhang Z. Autocrine CTHRC1 activates hepatic stellate cells and promotes liver fibrosis by activating TGF-β signaling. EBioMedicine. 2019; 40:43–55. PMID: 30639416.
Article27. Zhou H, Su L, Liu C, Li B, Li H, Xie Y, Sun D. CTHRC1 may serve as a prognostic biomarker for hepatocellular carcinoma. Onco Targets Ther. 2019; 12:7823–7831. PMID: 31576140.28. Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005; 16:159–178. PMID: 15863032.
Article29. Papagerakis P, Berdal A, Mesbah M, Peuchmaur M, Malaval L, Nydegger J, Simmer J, Macdougall M. Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone. 2002; 30:377–385. PMID: 11856645.
Article30. Suzuki S, Haruyama N, Nishimura F, Kulkarni AB. Dentin sialophosphoprotein and dentin matrix protein-1: two highly phosphorylated proteins in mineralized tissues. Arch Oral Biol. 2012; 57:1165–1175. PMID: 22534175.
Article31. Balic A, Mina M. Identification of secretory odontoblasts using DMP1-GFP transgenic mice. Bone. 2011; 48:927–937. PMID: 21172466.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dlx3 and Dlx5 Inhibit Adipogenic Differentiation of Human Dental Pulp Stem Cells
- Chios gum mastic enhance the proliferation and odontogenic differentiation of human dental pulp stem cells
- MiR-148a-3p Regulates the Invasion and Odontoblastic Differentiation of Human Dental Pulp Stem Cells via the Wnt1/β-Catenin Pathway
- A Trial of Screening of Genes Involved in Odontoblasts Differentiation from Human Dental Pulp Stem Cells
- NBCe1 Regulates Odontogenic Differentiation of Human Dental Pulp Stem Cells via NF-κB