Nutr Res Pract.
2015 Feb;9(1):11-16. 10.4162/nrp.2015.9.1.11.
Inhibitory activities of Perilla frutescens britton leaf extract against the growth, migration, and adhesion of human cancer cells
- Affiliations
-
- 1Department of Food and Nutrition, Chungbuk National University, 52 Naesudong-ro, Heungdeok-gu, Chungbuk, 361-763, Korea. jujih@chungbuk.ac.kr
- KMID: 2313803
- DOI: http://doi.org/10.4162/nrp.2015.9.1.11
Abstract
- BACKGROUND/OBJECTIVES
Perilla frutescens Britton leaves are a commonly consumed vegetable in different Asian countries including Korea. Cancer is a major cause of human death worldwide. The aim of the current study was to investigate the inhibitory effects of ethanol extract of perilla leaf (PLE) against important characteristics of cancer cells, including unrestricted growth, resisted apoptosis, and activated metastasis, using human cancer cells.
MATERIALS/METHODS
Two human cancer cell lines were used in this study, HCT116 colorectal carcinoma cells and H1299 non-small cell lung carcinoma cells. Assays using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide were performed for measurement of cell growth. Soft agar and wound healing assays were performed to determine colony formation and cell migration, respectively. Nuclear staining and cell cycle analysis were performed for assessment of apoptosis. Fibronectin-coated plates were used to determine cell adhesion.
RESULTS
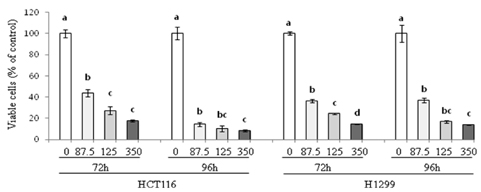
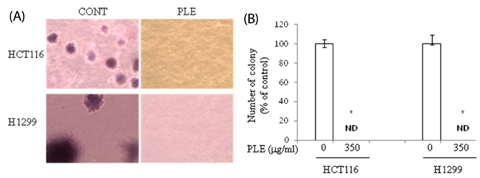
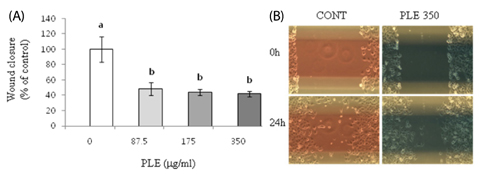
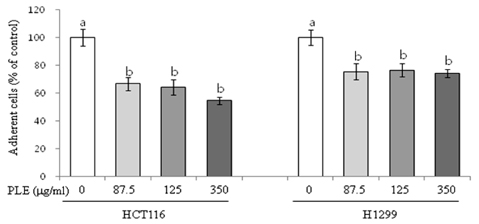
Treatment of HCT116 and H1299 cells with PLE resulted in dose-dependent inhibition of growth by 52-92% (at the concentrations of 87.5, 175, and 350 microg/ml) and completely abolished the colony formation in soft agar (at the concentration of 350 microg/ml). Treatment with PLE at the 350 microg/ml concentration resulted in change of the nucleus morphology and significantly increased sub-G1 cell population in both cells, indicating its apoptosis-inducing activity. PLE at the concentration range of 87.5 to 350 microg/ml was also effective in inhibiting the migration of H1299 cells (by 52-58%) and adhesion of both HCT116 and H1299 cells (by 25-46%).
CONCLUSIONS
These results indicate that PLE exerts anti-cancer activities against colon and lung cancers in vitro. Further studies are needed in order to determine whether similar effects are reproduced in vivo.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59:225–249.
Article2. Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003; 3:768–780.
Article3. Miller PE, Snyder DC. Phytochemicals and cancer risk: a review of the epidemiological evidence. Nutr Clin Pract. 2012; 27:599–612.4. Seo WH, Baek HH. Characteristic aroma-active compounds of Korean perilla (Perilla frutescens Britton) leaf. J Agric Food Chem. 2009; 57:11537–11542.
Article5. Meng L, Lozano YF, Gaydou EM, Li B. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules. 2008; 14:133–140.
Article6. Ueda H, Yamazaki M. Inhibition of tumor necrosis factor-alpha production by orally administering a perilla leaf extract. Biosci Biotechnol Biochem. 1997; 61:1292–1295.
Article7. Ueda H, Yamazaki M. Anti-inflammatory and anti-allergic actions by oral administration of a perilla leaf extract in mice. Biosci Biotechnol Biochem. 2001; 65:1673–1675.
Article8. Kim MK, Lee HS, Kim EJ, Won NH, Chi YM, Kim BC, Lee KW. Protective effect of aqueous extract of Perilla frutescens on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem Toxicol. 2007; 45:1738–1744.
Article9. Makino T, Furuta A, Fujii H, Nakagawa T, Wakushima H, Saito K, Kano Y. Effect of oral treatment of Perilla frutescens and its constituents on type-I allergy in mice. Biol Pharm Bull. 2001; 24:1206–1209.
Article10. Liu JY, Chen YC, Lin CH, Kao SH. Perilla frutescens leaf extract inhibits mite major allergen Der p 2-induced gene expression of pro-allergic and pro-inflammatory cytokines in human bronchial epithelial cell BEAS-2B. PLoS One. 2013; 8:e77458.
Article11. Makino T, Furuta Y, Wakushima H, Fujii H, Saito K, Kano Y. Antiallergic effect of Perilla frutescens and its active constituents. Phytother Res. 2003; 17:240–243.12. Kim MJ, Kim HK. Perilla leaf extract ameliorates obesity and dyslipidemia induced by high-fat diet. Phytother Res. 2009; 23:1685–1690.
Article13. Kwak CS, Yeo EJ, Moon SC, Kim YW, Ahn HJ, Park SC. Perilla leaf, Perilla frutescens, induces apoptosis and G1 phase arrest in human leukemia HL-60 cells through the combinations of death receptor-mediated, mitochondrial, and endoplasmic reticulum stress-induced pathways. J Med Food. 2009; 12:508–517.
Article14. Lin CS, Kuo CL, Wang JP, Cheng JS, Huang ZW, Chen CF. Growth inhibitory and apoptosis inducing effect of Perilla frutescens extract on human hepatoma HepG2 cells. J Ethnopharmacol. 2007; 112:557–567.
Article15. Ueda H, Yamazaki C, Yamazaki M. Inhibitory effect of Perilla leaf extract and luteolin on mouse skin tumor promotion. Biol Pharm Bull. 2003; 26:560–563.
Article16. Jeon IH, Kim HS, Kang HJ, Lee HS, Jeong SI, Kim SJ, Jang SI. Anti-inflammatory and antipruritic effects of luteolin from Perilla (P. frutescens L.) leaves. Molecules. 2014; 19:6941–6951.
Article17. Ueda H, Yamazaki C, Yamazaki M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol Pharm Bull. 2002; 25:1197–1202.
Article18. Osakabe N, Yasuda A, Natsume M, Sanbongi C, Kato Y, Osawa T, Yoshikawa T. Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in D-galactosamine (D-GalN)-sensitized mice. Free Radic Biol Med. 2002; 33:798–806.
Article19. Osakabe N, Yasuda A, Natsume M, Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004; 25:549–557.
Article20. Sanbongi C, Takano H, Osakabe N, Sasa N, Natsume M, Yanagisawa R, Inoue KI, Sadakane K, Ichinose T, Yoshikawa T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin Exp Allergy. 2004; 34:971–977.
Article21. Takano H, Osakabe N, Sanbongi C, Yanagisawa R, Inoue K, Yasuda A, Natsume M, Baba S, Ichiishi E, Yoshikawa T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp Biol Med (Maywood). 2004; 229:247–254.
Article22. Banno N, Akihisa T, Tokuda H, Yasukawa K, Higashihara H, Ukiya M, Watanabe K, Kimura Y, Hasegawa J, Nishino H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci Biotechnol Biochem. 2004; 68:85–90.
Article23. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674.
Article24. Lambert JD, Lu G, Lee MJ, Hu J, Ju J, Yang CS. Inhibition of lung cancer growth in mice by dietary mixed tocopherols. Mol Nutr Food Res. 2009; 53:1030–1035.
Article25. Irons R, Tsuji PA, Carlson BA, Ouyang P, Yoo MH, Xu XM, Hatfield DL, Gladyshev VN, Davis CD. Deficiency in the 15-kDa selenoprotein inhibits tumorigenicity and metastasis of colon cancer cells. Cancer Prev Res (Phila). 2010; 3:630–639.
Article26. Toton E, Ignatowicz E, Bernard MK, Kujawski J, Rybczynska M. Evaluation of apoptotic activity of new condensed pyrazole derivatives. J Physiol Pharmacol. 2013; 64:115–123.27. Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005; 5:876–885.
Article28. Florent M, Godard T, Ballet JJ, Gauduchon P, Sola B. Detection by the comet assay of apoptosis induced in lymphoid cell lines after growth factor deprivation. Cell Biol Toxicol. 1999; 15:185–192.29. Ju J, Kwak Y, Hao X, Yang CS. Inhibitory effects of calcium against intestinal cancer in human colon cancer cells and Apc(Min/+) mice. Nutr Res Pract. 2012; 6:396–404.
Article30. Matsuura N, Miyamae Y, Yamane K, Nagao Y, Hamada Y, Kawaguchi N, Katsuki T, Hirata K, Sumi S, Ishikawa H. Aged garlic extract inhibits angiogenesis and proliferation of colorectal carcinoma cells. J Nutr. 2006; 136:842S–846S.
Article31. Hotz MA, Gong J, Traganos F, Darzynkiewicz Z. Flow cytometric detection of apoptosis: comparison of the assays of in situ DNA degradation and chromatin changes. Cytometry. 1994; 15:237–244.
Article32. Li K, Zhu ZC, Liu YJ, Liu JW, Wang HT, Xiong ZQ, Shen X, Hu ZL, Zheng J. ZFX knockdown inhibits growth and migration of non-small cell lung carcinoma cell line H1299. Int J Clin Exp Pathol. 2013; 6:2460–2467.33. Tang J, Zhang L, She X, Zhou G, Yu F, Xiang J, Li G. Inhibiting CD164 expression in colon cancer cell line HCT116 leads to reduced cancer cell proliferation, mobility, and metastasis in vitro and in vivo. Cancer Invest. 2012; 30:380–389.
Article34. Dolfi SC, Yang Z, Lee MJ, Guan F, Hong J, Yang CS. Inhibitory effects of different forms of tocopherols, tocopherol phosphates, and tocopherol quinones on growth of colon cancer cells. J Agric Food Chem. 2013; 61:8533–8540.
Article35. Wang X, Wang Q, Ives KL, Evers BM. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin Cancer Res. 2006; 12:5346–5355.
Article36. Lu G, Xiao H, Li GX, Picinich SC, Chen YK, Liu A, Lee MJ, Loy S, Yang CS. A gamma-tocopherol-rich mixture of tocopherols inhibits chemically induced lung tumorigenesis in A/J mice and xenograft tumor growth. Carcinogenesis. 2010; 31:687–694.
Article37. Ballet F, Petit J, Poupon R, Darnis F. Soft agar clonogenic assay for predicting chemosensitivity of human tumor cells from malignant effusions. Biomedicine. 1981; 35:177–178.38. Shu L, Cheung KL, Khor TO, Chen C, Kong AN. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010; 29:483–502.
Article39. Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008; 8:634–646.
Article40. Wang Y, Huang X, Han J, Zheng W, Ma W. Extract of Perilla frutescens inhibits tumor proliferation of HCC via PI3K/AKT signal pathway. Afr J Tradit Complement Altern Med. 2012; 10:251–257.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Loquat (Eriobotrya japonica) extracts suppress the adhesion, migration and invasion of human breast cancer cell line
- Anti-Helicobacter pylori activity of acomplex mixture of Lactobacillus paracasei HP7 including the extract of Perilla frutescens var. acuta and Glycyrrhiza glabra
- Anti-hyperglycemic effects and signaling mechanism of Perilla frutescens sprout extract
- Inhibition of Proinflammatory Cytokine Generation in Lung Inflammation by the Leaves of Perilla frutescens and Its Constituents
- Inhibitory Effects on Oral Microbial Activity and Production of Lipopolysaccharides-Induced Pro-Inflammatory Mediators in Raw264.7 Macrophages of Ethanol Extract of Perilla flutescens (L.) Britton