Nutr Res Pract.
2014 Dec;8(6):655-661. 10.4162/nrp.2014.8.6.655.
Arctiin inhibits adipogenesis in 3T3-L1 cells and decreases adiposity and body weight in mice fed a high-fat diet
- Affiliations
-
- 1Department of Food and Nutrition, College of Human Ecology, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul 130-701, Korea. jchung@khu.ac.kr
- KMID: 2313792
- DOI: http://doi.org/10.4162/nrp.2014.8.6.655
Abstract
- BACKGROUND/OBJECTIVES
The purpose of this study was to examine the effects and associated mechanisms of arctiin, a lignan compound found in burdock, on adipogenesis in 3T3-L1 cells. Also, the effects of arctiin supplementation in obese mice fed a high-fat diet on adiposity were examined.
MATERIALS/METHODS
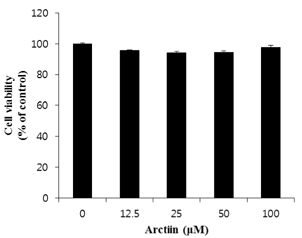
3T3-L1 cells were treated with arctiin (12.5 to 100 microM) during differentiation for 8 days. The accumulation of lipid droplets was determined by Oil Red O staining and intracellular triglyceride contents. The expressions of genes related to adipogenesis were measured by real-time RT-PCR and Western blot analyses. For in vivo study, C57BL/6J mice were first fed either a control diet (CON) or high-fat diet (HF) to induce obesity, and then fed CON, HF, or HF with 500 mg/kg BW arctiin (HF + AC) for four weeks.
RESULTS
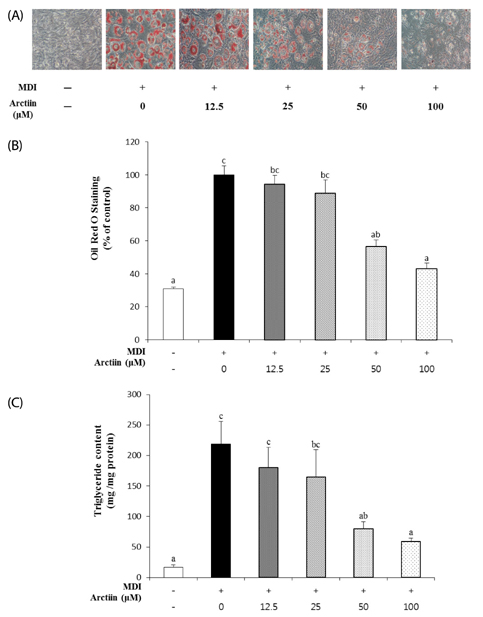
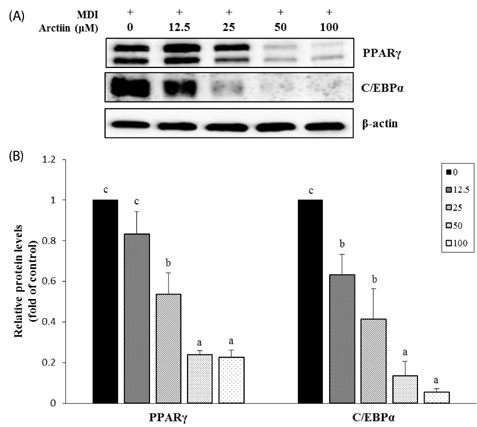
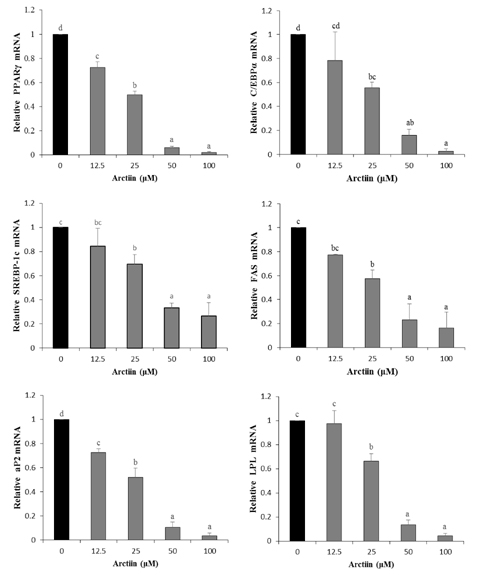
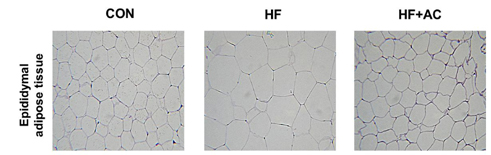
Arctiin treatment to 3T3-L1 pre-adipocytes markedly decreased adipogenesis in a dose-dependent manner. The arctiin treatment significantly decreased the protein levels of the key adipogenic regulators PPARgamma and C/EBPalpha, and also significantly inhibited the expression of SREBP-1c, fatty acid synthase, fatty acid-binding protein and lipoprotein lipase. Also, arctiin greatly increased the phosphorylation of AMP-activated protein kinase (AMPK) and its downstream target phosphorylated-acetyl CoA carboxylase. Furthermore, administration of arctiin significantly decreased the body weight in obese mice fed with the high-fat diet. The epididymal, perirenal or total visceral adipose tissue weights of mice were all significantly lower in the HF + AC than in the HF. Arctiin administration also decreased the sizes of lipid droplets in the epididymal adipose tissue.
CONCLUSIONS
Arctiin inhibited adipogenesis in 3T3-L1 adipocytes through the inhibition of PPARgamma and C/EBPalpha and the activation of AMPK signaling pathways. These findings suggest that arctiin has a potential benefit in preventing obesity.
Keyword
MeSH Terms
-
3T3-L1 Cells*
Adenylate Kinase
Adipocytes
Adipogenesis*
Adipose Tissue
Adiposity*
AMP-Activated Protein Kinases
Animals
Blotting, Western
Body Weight*
Diet
Diet, High-Fat*
Intra-Abdominal Fat
Lipoprotein Lipase
Mice*
Mice, Obese
Obesity
Phosphorylation
PPAR gamma
Sterol Regulatory Element Binding Protein 1
Triglycerides
Weights and Measures
AMP-Activated Protein Kinases
Adenylate Kinase
Lipoprotein Lipase
PPAR gamma
Sterol Regulatory Element Binding Protein 1
Figure
Cited by 1 articles
-
Effects of developmental iron deficiency and post-weaning iron repletion on the levels of iron transporter proteins in rats
Sugyoung Oh, Pill-kyung Shin, Jayong Chung
Nutr Res Pract. 2015;9(6):613-618. doi: 10.4162/nrp.2015.9.6.613.
Reference
-
1. World Health Organization. Obesity and overweight [Internet]. [place unknown]: World Health Organization;2014. cited 2014 May. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.2. Konige M, Wang H, Sztalryd C. Role of adipose specific lipid droplet proteins in maintaining whole body energy homeostasis. Biochim Biophys Acta. 2014; 1842:393–401.
Article3. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006; 444:881–887.
Article4. Kopelman PG. Obesity as a medical problem. Nature. 2000; 404:635–643.
Article5. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. American Heart Association. National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004; 109:433–438.6. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000; 106:473–481.
Article7. Siersbaek R, Nielsen R, Mandrup S. PPARgamma in adipocyte differentiation and metabolism--novel insights from genome-wide studies. FEBS Lett. 2010; 584:3242–3249.
Article8. Bastie C, Holst D, Gaillard D, Jehl-Pietri C, Grimaldi PA. Expression of peroxisome proliferator-activated receptor PPARdelta promotes induction of PPARgamma and adipocyte differentiation in 3T3C2 fibroblasts. J Biol Chem. 1999; 274:21920–21925.
Article9. He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003; 100:15712–15717.10. Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. Impaired energy homeostasis in C/EBP alpha knockout mice. Science. 1995; 269:1108–1112.11. Lin FT, Lane MD. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci U S A. 1994; 91:8757–8761.
Article12. Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999; 3:151–158.
Article13. Farmer SR. Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond). 2005; 29:Suppl 1. S13–S16.14. White UA, Stephens JM. Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol. 2010; 318:10–14.
Article15. Bae CR, Park YK, Cha YS. Quercetin-rich onion peel extract suppresses adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 adipocytes. J Sci Food Agric. 2014; Forthcoming.
Article16. Hayashi K, Narutaki K, Nagaoka Y, Hayashi T, Uesato S. Therapeutic effect of arctiin and arctigenin in immunocompetent and immunocompromised mice infected with influenza A virus. Biol Pharm Bull. 2010; 33:1199–1205.
Article17. Matsuzaki Y, Koyama M, Hitomi T, Yokota T, Kawanaka M, Nishikawa A, Germain D, Sakai T. Arctiin induces cell growth inhibition through the down-regulation of cyclin D1 expression. Oncol Rep. 2008; 19:721–727.
Article18. Wu JG, Wu JZ, Sun LN, Han T, Du J, Ye Q, Zhang H, Zhang YG. Ameliorative effects of arctiin from Arctium lappa on experimental glomerulonephritis in rats. Phytomedicine. 2009; 16:1033–1041.
Article19. Lee S, Shin S, Kim H, Han S, Kim K, Kwon J, Kwak JH, Lee CK, Ha NJ, Yim D, Kim K. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-kappaB pathways. J Inflamm (Lond). 2011; 8:16.20. Tice A, Ruckle JE, Sultan OS, Kemble S. Access to care: the physician's perspective. Hawaii Med J. 2011; 70:33–38.21. Kim WK, Kang NE, Kim MH, Ha AW. Peanut sprout ethanol extract inhibits the adipocyte proliferation, differentiation, and matrix metalloproteinases activities in mouse fibroblast 3T3-L1 preadipocytes. Nutr Res Pract. 2013; 7:160–165.
Article22. Yamasaki M, Ogawa T, Wang L, Katsube T, Yamasaki Y, Sun X, Shiwaku K. Anti-obesity effects of hot water extract from Wasabi (Wasabia japonica Matsum.) leaves in mice fed high-fat diets. Nutr Res Pract. 2013; 7:267–272.
Article23. Yao X, Zhu F, Zhao Z, Liu C, Luo L, Yin Z. Arctigenin enhances chemosensitivity of cancer cells to cisplatin through inhibition of the STAT3 signaling pathway. J Cell Biochem. 2011; 112:2837–2849.
Article24. Awale S, Lu J, Kalauni SK, Kurashima Y, Tezuka Y, Kadota S, Esumi H. Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res. 2006; 66:1751–1757.
Article25. Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000; 130:3122S–3126S.
Article26. Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cell Biol. 2013; 92:229–236.
Article27. Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, Bapat P, Kwun I, Shen CL. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. 2014; 25:1–18.
Article28. Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996; 10:1096–1107.
Article29. Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc Natl Acad Sci U S A. 1998; 95:4333–4337.30. Ceddia RB. The role of AMP-activated protein kinase in regulating white adipose tissue metabolism. Mol Cell Endocrinol. 2013; 366:194–203.
Article31. Daval M, Foufelle F, Ferré P. Functions of AMP-activated protein kinase in adipose tissue. J Physiol. 2006; 574:55–62.
Article32. Habinowski SA, Witters LA. The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 2001; 286:852–856.
Article33. Lee H, Kang R, Bae S, Yoon Y. AICAR, an activator of AMPK, inhibits adipogenesis via the WNT/beta-catenin pathway in 3T3-L1 adipocytes. Int J Mol Med. 2011; 28:65–71.34. Vingtdeux V, Chandakkar P, Zhao H, Davies P, Marambaud P. Small-molecule activators of AMP-activated protein kinase (AMPK), RSVA314 and RSVA405, inhibit adipogenesis. Mol Med. 2011; 17:1022–1030.
Article35. Kuo DH, Hung MC, Hung CM, Liu LM, Chen FA, Shieh PC, Ho CT, Way TD. Body weight management effect of burdock (Arctium lappa L.) root is associated with the activation of AMP-activated protein kinase in human HepG2 cells. Food Chem. 2012; 134:1320–1326.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The protective effects of steamed ginger on adipogenesis in 3T3-L1 cells and adiposity in diet-induced obese mice
- Antiproliferative properties of luteolin against chemically induced colon cancer in mice fed on a high-fat diet and colorectal cancer cells grown in adipocyte-derived medium
- Capsanthin Inhibits both Adipogenesis in 3T3-L1 Preadipocytes and Weight Gain in High-Fat Diet-Induced Obese Mice
- Expression of eotaxin in 3T3-L1 adipocytes and the effects of weight loss in high-fat diet induced obese mice
- Pear pomace ethanol extract improves insulin resistance through enhancement of insulin signaling pathway without lipid accumulation