Nutr Res Pract.
2010 Oct;4(5):351-355.
Significance of p27(kip1) as potential biomarker for intracellular oxidative status
- Affiliations
-
- 1School of Applied Biosciences, Kyungpook National University, 1370 Sankyuk-dong, Buk-gu, Daegu 702-701, Korea. vision@knu.ac.kr
- 2Department of Food Science and Nutrition, Sookmyung Women's University, Seoul 140-702, Korea.
- 3Department of Life Science, Dongguk University, Seoul 100-715, Korea.
Abstract
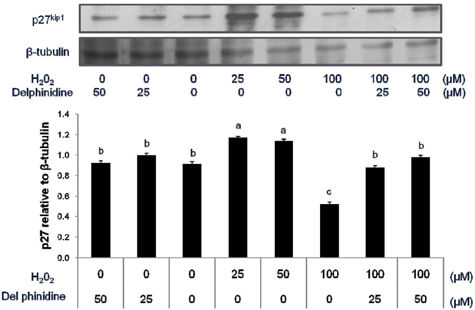
- Our previous proteomic study demonstrated that oxidative stress and antioxidant delphinidin regulated the cellular level of p27(kip1) (referred to as p27) as well as some heat shock proteins in human colon cancer HT 29 cells. Current study was conducted to validate and confirm the regulation of these proteins using both in vitro and in vivo systems. The level of p27 was decreased by hydrogen peroxide in a dose-dependent manner in human colon carcinoma HCT 116 (p53-positive) cells while it was increased upon exposure to hydrogen peroxide in HT 29 (p53-negative) cells. However, high concentration of hydrogen peroxide (100 micrometer) downregulated p27 in both cell lines, but delphindin, one of antioxidative anthocyanins, enhanced the level of p27 suppressed by 100 micrometer hydrogen peroxide. ICR mice were injected with varying concentrations of hydrogen peroxide, delphinidin and both. Western blot analysis for the mouse large intestinal tissue showed that the expression of p27 was upregulated by 25 mg/kg BW hydrogen peroxide. To investigate the association of p27 regulation with hypoxia-inducible factor 1-beta (HIF-1beta), the level of p27 was analyzed in wild-type mouse hepatoma hepa1c1c7 and Aryl Hydrocarbon Nuclear Translocator (arnt, HIF-1beta)-defective mutant BPRc1 cells in the absence and presence of hydrogen peroxide and delphinidin. While the level of p27 was responsive to hydrogen peroxide and delphinidin, it remained unchanged in BPRc1, suggesting that the regulation of p27 requires functional HIF-1beta. We also found that hydrogen peroxide and delphinidin affected PI3K/Akt/mTOR signaling pathway which is one of upstream regulators of HIFs. In conclusion, hydrogen peroxide and antioxidant delphinidin seem to regulate intracellular level of p27 through regulating HIF-1 level which is, in turn, governed by its upstream regulators comprising of PI3K/Akt/mTOR signaling pathway. The results should also encourage further study for the potential of p27 as a biomarker for intracellular oxidative or antioxidant status.
Keyword
MeSH Terms
-
Animals
Anthocyanins
Aryl Hydrocarbon Receptor Nuclear Translocator
Blotting, Western
Carcinoma, Hepatocellular
Cell Line
Colon
Colonic Neoplasms
Heat-Shock Proteins
HT29 Cells
Humans
Hydrogen Peroxide
Mice
Mice, Inbred ICR
Oxidative Stress
Proteins
Anthocyanins
Aryl Hydrocarbon Receptor Nuclear Translocator
Heat-Shock Proteins
Hydrogen Peroxide
Proteins
Figure
Reference
-
1. Haddad JE, Olver RE, Land SC. Antioxidant/Pro-oxidant equilibrium regulates HIF-1 and NF-κB redox sensitivity. J Biol Chem. 2000. 275:21130–21139.
Article2. Paron I, D'Elia A, D'Ambrosio C, Scaloni A, D'Aurizio F, Prescott A, Damante G, Tell G. A proteomic approach to identify early molecular targets of oxidative stress in human epithelial lens cells. Biochem J. 2004. 378:929–937.
Article3. Smith J, Ladi E, Proschel MM, Noble M. Redox state is a central regulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000. 97:10032–10037.
Article4. Jang CH, Lee IA, Ha YR, Lim J, Sung MK, Lee SJ, Kim JS. PGK1 induction by hydrogen peroxide treatment is suppressed by antioxidants in human colon carcinoma cells. Biosci Biotechnol Biochem. 2008. 72:1799–1808.
Article5. Abukhdeir AM, Park BH. P21 and p27: roles in carcinogenesis and drug resistance. Expert Rev Mol Med. 2008. 10:e19.
Article6. Green SL, Freiberg RA, Giaccia AJ. p21(Cip1) and p27(Kip1) regulate cell cycle reentry after hypoxic stress but are not necessary for hypoxia-induced arrest. Mol Cell Biol. 2001. 21:1196–1206.
Article7. Hackenbeck T, Knaup KX, Schietke R, Schödel J, Willam C, Wu X, Warnecke C, Eckardt KU, Wiesener MS. HIF-1 or HIF-2 induction is sufficient to achieve cell cycle arrest in NIH3T3 mouse fibroblasts independent from hypoxia. Cell Cycle. 2009. 8:1386–1395.
Article8. Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000. 88:1474–1480.
Article9. Goda N, Ryan HE, Khadivi B, McNulty W, Rickert RC, Johnson RS. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol. 2003. 23:359–369.
Article10. Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990. 4:2587–2597.
Article11. Shackelford RE, Kaufmann WK, Paules RS. Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med. 2000. 28:1387–1404.12. Massagué J. G1 cell-cycle control and cancer. Nature. 2004. 432:298–306.
Article13. Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999. 13:1501–1512.
Article14. Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumor angiogenesis. Nature. 1998. 394:485–490.
Article15. Gardner LB, Li Q, Park MS, Flanagan WM, Semenza GL, Dang CV. Hypoxia inhibits G1/S transition through regulation of p27 expression. J Biol Chem. 2001. 276:7919–7926.
Article16. Mack FA, Patel JH, Biju MP, Haase VH, Simon MC. Decreased growth of Vhl-/- fibrosarcomas is associated with elevated levels of cyclin kinase inhibitors p21 and p27. Mol Cell Biol. 2005. 25:4565–4578.
Article17. Horrée N, Gort EH, van der Groep P, Heintz AP, Vooijs M, van Diest PJ. Hypoxia-inducible factor 1 alpha is essential for hypoxic p27 induction in endometrioid endometrial carcinoma. J Pathol. 2008. 214:38–45.
Article18. Semenza GL. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol. 2009. 19:12–16.
Article19. Wang GL, Jiang BH, Rue EA. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995. 92:5510–5514.
Article20. Semenza GL. HIF-1 and mechanism of hypoxia sensing. Curr Opin Cell Biol. 2001. 13:167–171.21. Ardyanto TD, Osaki M, Tokuyasu N, Nagahama Y, Ito H. CoCl2-induced HIF-1alpha expression correlates with proliferation and apoptosis in MKN-1 cells: a possible role for the PI3K/Akt pathway. Int J Oncol. 2006. 29:549–555.22. Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008. 33:526–534.
Article23. Qutub AA, Popel AS. Reactive oxygen species regulate hypoxia-inducible factor 1alpha differentially in cancer and ischemia. Mol Cell Biol. 2008. 28:5106–5119.
Article24. Galanis A, Pappa A, Giannakakis A, Lanitis E, Dangaj D, Sandaltzopoulos R. Reactive oxygen species and HIF-1 signalling in cancer. Cancer Lett. 2008. 266:12–20.
Article25. Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008. 8:425–437.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Relevance of Women's Diseases, Jun Activation-domain Binding Protein 1 (JAB1) and p27(kip1)
- Significance of the Expression of p27(Kip1) Protein in Human Breast Cancer
- Expression of p27kip1, Cyclin D1 and p53 Protein in Ductal Carcinoma In Situ of the Breast
- The Expression of p27(kip1) and p57(kip2) in Mouse Endometrium
- Prognostic Implications of Cyclin B1, p34cdc2, p27(Kip1) and p53 Expression in Gastric Cancer