Nutr Res Pract.
2007 Jun;1(2):113-119.
Zinc deficiency negatively affects alkaline phosphatase and the concentration of Ca, Mg and P in rats
- Affiliations
-
- 1Department of Food Science and Nutrition, Andong National University, Andong, Gyeongpook 760-749, Korea. iskwun@andong.ac.kr
- 2Central Laboratory Division for Instrumental Analysis, Kyungbook National University, Daegu 702-701, Korea.
- 3School of Dentistry, Kyungbook National University, Daegu 702-701, Korea.
- 4Cellular Integrity Division, Rowett Research Institute, Aberdeen, Scotland, United Kingdom.
Abstract
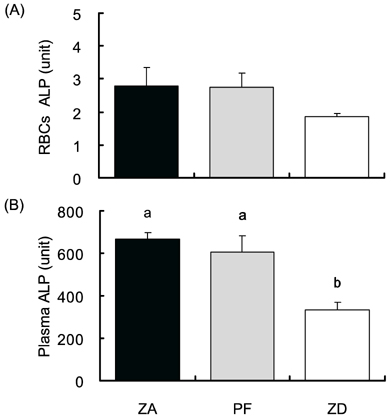
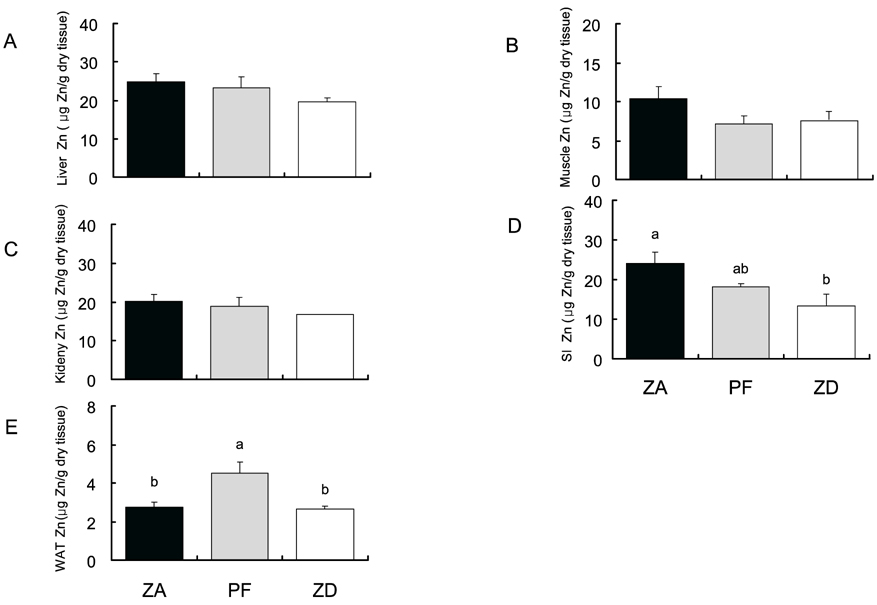
- Zn is an essential nutrient that is required in humans and animals for many physiological functions, including immune and antioxidant function, growth, and reproduction. The present study evaluated whether Zn deficiency would negatively affect bone-related enzyme, ALP, and other bone-related minerals (Ca, P and Mg) in rats. Thirty Sprague Dawley rats were assigned to one of the three different Zn dietary groups, such as Zn adequate (ZA, 35 mg/kg), pair fed (PF, 35 mg/kg), Zn deficient (ZD, 1 mg/kg) diet, and fed for 10 weeks. Food intake and body weight were measured daily and weekly, respectively. ALP was measured by spectrophotometry and mineral contents were measured by inductively coupled plasma-mass spectrophotometer (ICP-MS). Zn deficient rats showed decreased food intake and body weight compared with Zn adequate rats (p<0.05). Zn deficiency reduced ALP activity in blood (RBC, plasma) and the tissues (liver, kidney and small intestine) (p<0.05). Also, Zn deficiency reduced mineral concentrations in rat tissues (Ca for muscle and liver, and Mg for muscle and liver) (p<0.05). The study results imply the requirement of proper Zn nurture for maintaining bone growth and formation.
MeSH Terms
Figure
Reference
-
1. Bessey OA, Lowry OH, Brock MJ. A method for the rapid determination of alkaline phosphatase with fibecubic millimetres of serum. J Biol Chem. 1946. 164:321–329.
Article2. Bouglé DL, Sabatier JP, Guaydier-Souquières G, Guillon-Metz F, Laroche D, Jauzac P, Bureau F. Zinc status and bone mineralization in adolescent girls. J Trace Elem Med Biol. 2004. 18:17–21.3. Chesters JK, Quarterman J. Effects of zinc deficiency on food intake and feeding patterns of rats. Br J Nutr. 1970. 24:1061–1069.
Article4. Doherty CP, Crofton PM, Sarkar MA, Shakur MS, Wade JC, Kelnar CJ, Elmlinger MW, Ranke MB, Cutting WA. Malnutrition, zinc supplementation and catch-up growth: changes in insulin-like growth factor I, its binding proteins, bone formation and collagen turnover. Clin Endocrinol (Oxf). 2002. 57:391–399.
Article5. Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000. 289:1501–1504.
Article6. Eberle J, Schmidmayer S, Erben RG, Stangassinger M, Roth HP. Skeletal effects of zinc deficiency in growing rats. J Trace Elem Med Biol. 1999. 13:21–26.
Article7. Elmstachl S, Gullberg B, Janzon L, Johnell O, Elmstahl B. Increased incidence of fractures in middle-aged and elderly men with low intake of phosporus and zinc. Osteoporos Int. 1998. 8:333–340.8. Frost HM. Tetracycline-based histological analysis of bone remodeling. Calcif Tissue Res. 1969. 3:211–237.
Article9. Galdes A, Vallee BL. Sigel H, editor. Categories of zinc metalloenzymes. Metal ions in biological systems. 1983. New York. USA: Marcel Delkker;1–47.10. Giugiano R, Millward DJ. The effects of severe zinc deficiency on protein turnover in muscle and tymus. Br J Nutr. 1987. 57:139–155.
Article11. Hendy HA, Yousef MI, Naga NI. Effect of dietary zinc deficiency on hematological and biochemical parameters and concentrations of zinc, copper, and iron in growing rats. Toxicology. 2001. 167:163–170.
Article12. Holloway WR, Collier FM, Herbst RE, Hodge JM, Nicholson GC. Osteoblast-mediated effects of zinc on isolated rat osteoclasts: inhibition of bone resort ion and enhancement of osteoclast number. Bone. 1996. 12:137–142.13. Hosea HJ, Taylor CG, Wood T, Mollard R, Weiler HA. Zinc-deficient rats have more limited bone recovery during repletion than diet-restricted rats. Exp Biol Med (Maywood). 2004. 229:303–311.
Article14. Hurley LS, Gordon PR, Keen CL, Merkhofer L. Circadian variation in rat plasma zinc and rapid effect of dietary zinc deficiency. Proc Soc Exp Biol Med. 1982. 170:48–52.
Article15. Hyun TH, Barrett-connor E, Milne DB. Zinc intakes and plasma concentrations in men with osteoporosis: the Rancho Bernardo Study. Am J Clin Nutr. 2004. 80:715–721.
Article16. Karsdal MA, Martin TJ, Bollerslev J, Christiansen C, Henriksen K. Are nonresorbing osteoclasts sources of boe anabolic activity? J Bone Miner Res. 2007. 22:487–494.
Article17. Koshihara M, Masuyama R, Suzuk K. Reduction in dietary calcium/phosphorusratio reduces bone mass and strength in ovariectomized rats enhancing bone turnover. Biosci Biotechnol Biochem. 2005. 69:1970–1973.
Article18. Kwun IS, Cho YE, Lomeda RA, Kwon ST, Kim Y, Beattie JH. Zinc deficiency in rats decreases leptin expression independently of food intake and corticotrophin-releasing hormone in relation to food intake. Brit J Nutr. 2007. 97:[in press].19. Lee SL, Kwak EH, Kim Y, Choi JY, Kwon ST, Beattie JH, Kwun IS. Leptin gene expression and serum leptin levels in zinc deficiency: Implications for appetite regulation in rats. J Med Food. 2003. 6:281–290.
Article20. Lowry OB, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951. 193:265–275.21. Moonga BS, Dempster DW. Zinc is a potent inhibitor of osteoblastic bone resorption in vitro. J Bone Miner Res. 1995. 10:453–457.
Article22. National Research Council. Guide for the Care and Use of Laboratory Animals. 1996. Washington DC. USA: National Academy Press.23. O'Dell BL, Becker JK, Emery MP, Browining JD. Prodution and reversal of the neuromuscular pathology and related signs of zinc deficiency in guinea pigs. J Nutr. 1989. 119:196–201.24. Ovesen J, Moller-Madsen B, Thomsen JS, Danscher G, Mosekilde L. The positive effects of zinc on skeletal strength in growing rats. Bone. 2001. 29:565–570.
Article25. Peretz A, Papadopoulos T, Willems D, Hotimsky A, Michiels N, Siderova V, Bergmann P, Neve J. Zinc supplementation increases bone alkaline phosphatase in healthy men. J Trace Elem Med Biol. 2001. 15:175–178.
Article26. Rossi L, Migliaccio S, Corsi A, Marzia M, Bianco P, Teti A, Gambelli L, Cianfarani S, Paoletti F, Branca F. Reduced growth and skeletal changes in zinc-deficient growing rats and due to impaired growth plate activity and inanition. J Nutr. 2001. 131:1142–1146.
Article27. Roth HP. Development of Alimentary Zinc Deficiency in Growing Rats Is Retarded at Low Dietary Protein Levels. J Nutr. 2003. 133:2294–2301.
Article28. Sambrook P, Cooper C. Osteoporosis. Lancet. 2006. 367:2010–2018.
Article29. Scrimgeour AG, stahl CH, McClung JP, Marchitelli LJ, Young AJ. Moderate zinc deficiency negatively affects biomechanical properties of rat tibiae independently of body composition. J Nutr Biochem. 2007. 30:[Epub ahead of print].
Article30. Seco C, Revilla M, Hernández ER, Gervás J, González-Riola J, Villa LF, Rico H. Effects of zinc supplementation treadmill training exercise. J Bone Miner Res. 1998. 13:508–512.
Article31. Shinozaki T, Pritzker KP. Regulation of alkaline phosphatase: implications for calcium pyrophosphate dihydrate crystal dissolution and other alkaline phosphatase functions. J Rheumatol. 1996. 23:677–683.32. Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993. 14:424–442.
Article33. Suwarnasarn A, Wallwork JC, Lykken GI, Low FN, Sandstead HH. Epiphyseal plate development in the zinc-defecient rat. J Nutr. 1982. 112:1320–1380.34. Tang Z, Sahu SN, Khadeer MA, Bai G, Franklin RB, Gupta A. Overexpression of the ZIP1 zinc transporter induces an osteogenic phenotype in mesenchymal stem cells. Bone. 2006. 38:181–198.
Article35. Underwood EJ. The mineral nutrition of livestock. Commonwealth Agricultural Bureaux. 1981. Oxford: Oxford University Press.36. Valle BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993. 73:79–118.
Article37. Yamaguchi M, Oisgi H, Suketa Y. Zinc stimulation of bone protein synthesis in tissue culture. Activation of aminoacyl-tRNA sythetase. Biochem Pharmacol. 1988. 37:4075–4080.38. Yamaguchi M, Yamaguchi R. Action of zinc on bone metabolism in rats. Increase in alkaline phosphatase activity and DNA content. Biochem Pharmacol. 1986. 35:773–777.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Acrodermatitis Enteropathica with Normal Serum Zinc Level in a Breastfed Preterm Infant
- The Study in Vitamin D Concentration in the Blood for Infants with High Level of Alkaline Phosphatase
- Cellular zinc deficiency inhibits the mineralized nodule formation and downregulates bone-specific gene expression in osteoblastic MC3T3-E1 cells
- A study on the activity of alkaline phosphatase of rat oviduct during early embryonic development
- Studies on Alkaline Phosphatase Isoenzyme in the Serum and Organs of the Rat