Lab Anim Res.
2015 Sep;31(3):101-110. 10.5625/lar.2015.31.3.101.
Hepatotoxicity and nephrotoxicity of gallotannin-enriched extract isolated from Galla Rhois in ICR mice
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources and Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Department of Clinical Laboratory Science, College of Nursing and Healthcare Science, Dong-Eui University, Busan, Korea.
- KMID: 2312126
- DOI: http://doi.org/10.5625/lar.2015.31.3.101
Abstract
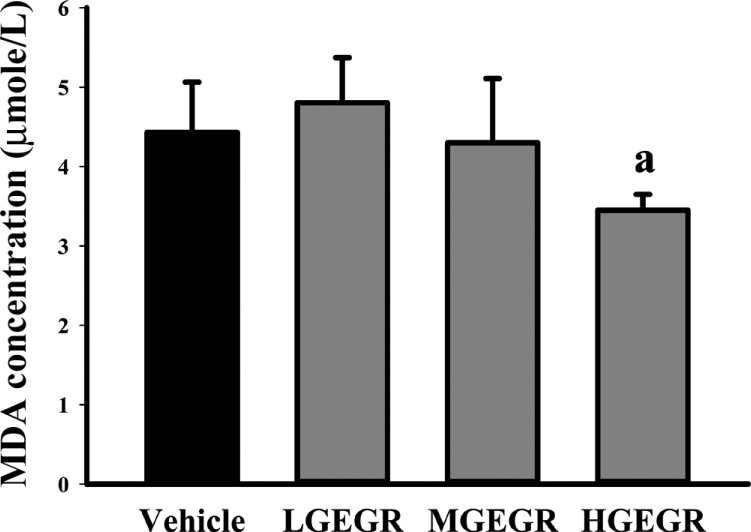
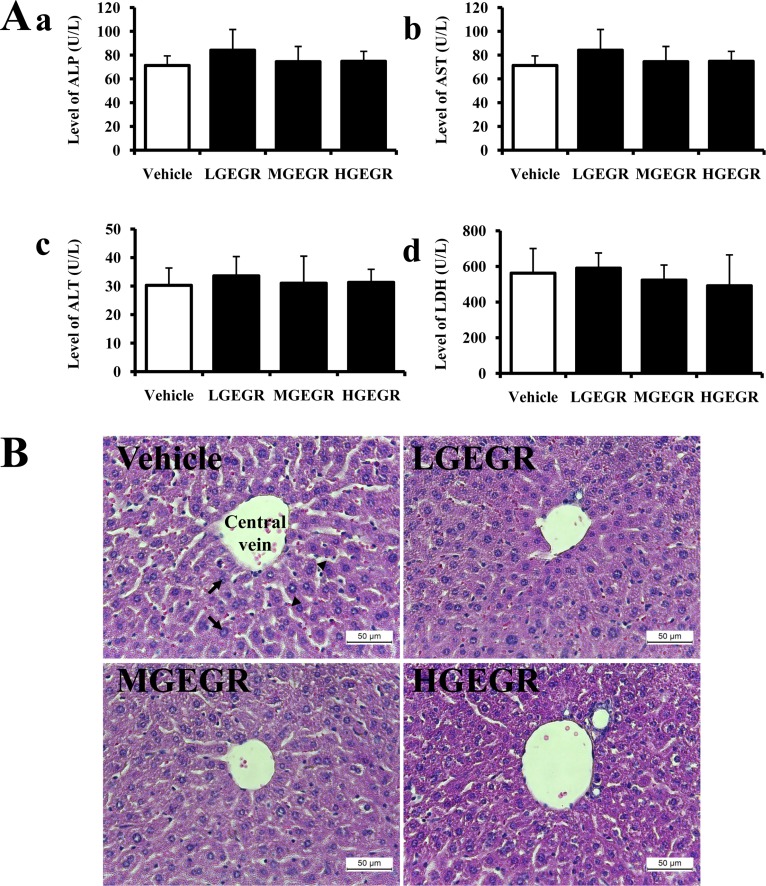
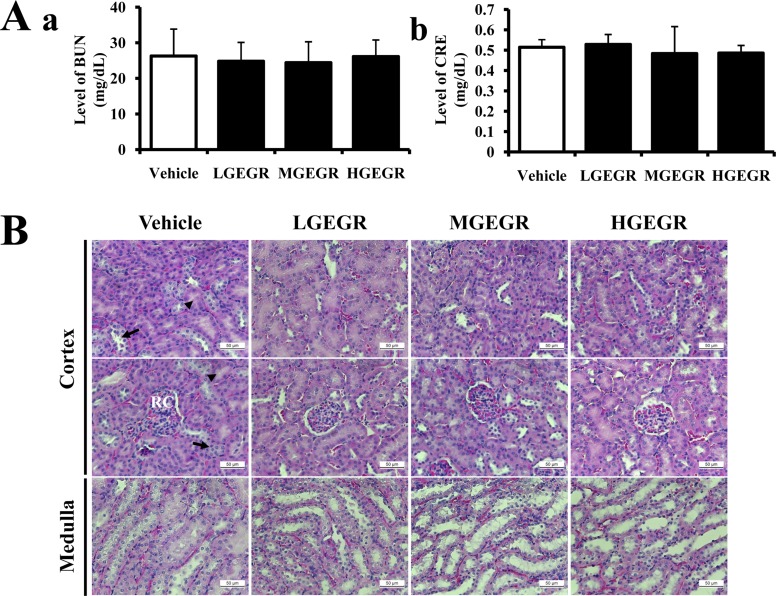
- To evaluate the hepatotoxicity and nephrotoxicity of Galla Rhois (GR) toward the liver and kidney of ICR mice, alterations in related markers including body weight, organ weight, urine composition, liver pathology and kidney pathology were analyzed after oral administration of 250, 500 and 1,000 mg/kg body weight/day of gallotannin-enriched extract of GR (GEGR) for 14 days. GEGR contained 68.7+/-2.5% of gallotannin, 25.3+/-0.9% of gallic acid and 4.4+/-0.1% of methyl gallate. Also, the level of malondialdehyde (MDA), a marker of lipid peroxidation, was decreased with 19% in the serum of high dose GEGR (HGEGR)-treated mice. The body and organ weight, clinical phenotypes, urine parameters and mice mortality did not differ among GEGR-treated groups and the vehicle-treated group. Furthermore, no significant increase was observed in alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), blood urea nitrogen (BUN) and the serum creatinine (Cr) in the GEGR-treated group relative to the vehicle-treated group. Moreover, the specific pathological features induced by most toxic compounds such as CCl4 were not observed upon liver and kidney histological analysis. Overall, the results of the present study suggest that GEGR does not induce any specific toxicity in liver and kidney organs of ICR at doses of 1,000 mg/kg body weight/day, indicating that this is no observed adverse effect level (NOAEL).
Keyword
MeSH Terms
-
Administration, Oral
Alanine Transaminase
Alkaline Phosphatase
Animals
Aspartate Aminotransferases
Blood Urea Nitrogen
Body Weight
Creatinine
Gallic Acid
Kidney
L-Lactate Dehydrogenase
Lipid Peroxidation
Liver
Malondialdehyde
Mice
Mice, Inbred ICR*
Mortality
No-Observed-Adverse-Effect Level
Organ Size
Pathology
Phenotype
Alanine Transaminase
Alkaline Phosphatase
Aspartate Aminotransferases
Creatinine
Gallic Acid
L-Lactate Dehydrogenase
Malondialdehyde
Figure
Reference
-
1. Lee SM, Lee DW, Park JD, Kim JI. Study on formation and development of gall in Rhus javanica. Korean J Appl Entomol. 1997; 36(1):83–87.2. Ren Z, Zhu B, Wang D, Ma E, Su D, Zhong Y. Comparative population structure of Chinese sumac aphid Schlechtendalia chinensis and its primary host-plant Rhus chinensis. Genetica. 2008; 132(1):103–112. PMID: 17503190.3. An RB, Oh H, Kim YC. Phenolic constituents of Galla Rhois with hepatoprotective effects on tacrine- and nitrofurantoin-induced cytotoxicity in Hep G2 cells. Biol Pharm Bull. 2005; 28(11):2155–2157. PMID: 16272710.
Article4. Song GY, Park BJ, Kim SH. Antithrombotic effect of Galla Rhois. Korean J Pharmacogn. 2002; 33(2):120–123.5. Shim YJ, Doo HK, Ahn SY, Kim YS, Seong JK, Park IS, Min BH. Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J Ethnopharmacol. 2003; 85(2-3):283–287. PMID: 12639753.6. Kim SH, Park HH, Lee S, Jun CD, Choi BJ, Kim SY, Kim SH, Kim DK, Park JS, Chae BS, Shin TY. The anti-anaphylactic effect of the gall of Rhus javanica is mediated through inhibition of histamine release and inflammatory cytokine secretion. Int Immunopharmacol. 2005; 5(13-14):1820–1829. PMID: 16275618.7. Ahn YJ, Lee CO, Kweon JH, Ahn JW, Park JH. Growth-inhibitory effects of Galla Rhois-derived tannins on intestinal bacteria. J Appl Microbiol. 1998; 84(3):439–443. PMID: 9721649.
Article8. Choi JG, Kang OH, Lee YS, Oh YC, Chae HS, Jang HJ, Kim JH, Sohn DH, Shin DW, Park H, Kwon DY. In vitro activity of methyl gallate isolated from galla rhois alone and in combination with ciprofloxacin against clinical isolates of salmonella. J Microbiol Biotechnol. 2008; 18(11):1848–1852. PMID: 19047831.9. Lee JJ, Bae JH, Kim DH, Lim JJ, Kim DG, Lee HJ, Min W, Rhee MH, Chang HH, Park H, Kim S. Intracellular replication inhibitory effects of Galla Rhois ethanol extract for Brucella abortus infection. J Ethnopharmacol. 2011; 138(2):602–609. PMID: 22008879.10. Lee JJ, Kim DH, Lim JJ, Kim DG, Min W, Kim GS, Lee HJ, Rhee MH, Park H, Kim SC, Chang HH, Kim S. Anticoccidial effect of supplemental dietary Galla Rhois against infection with Eimeria tenella in chickens. Avian Pathol. 2012; 41(4):403–407. PMID: 22834556.11. Chae HS, Kang OH, Choi JG, Oh YC, Lee YS, Brice OO, Chong MS, Lee KN, Shin DW, Kwon DY. Methyl gallate inhibits the production of interleukin-6 and nitric oxide via down-regulation of extracellular-signal regulated protein kinase in RAW 264.7 cells. Am J Chin Med. 2010; 38(5):973–983. PMID: 20821827.
Article12. Park PH, Hur J, Kim YC, An RB, Sohn DH. Involvement of heme oxygenase-1 induction in inhibitory effect of ethyl gallate isolated from Galla Rhois on nitric oxide production in RAW 264.7 macrophages. Arch Pharm Res. 2011; 34(9):1545–1552. PMID: 21975817.
Article13. Ata N, Oku T, Hattori M, Fujii H, Nakajima M, Saiki I. Inhibition by galloylglucose (GG6-10) of tumor invasion through extracellular matrix and gelatinase-mediated degradation of type IV collagens by metastatic tumor cells. Oncol Res. 1996; 8(12):503–511. PMID: 9160354.14. Xiang Q, Fan C, Xiao S, Pan H, Wang J, Zhao N, Tian J. Effect of gallnut extract on nasopharyngeal carcinoma 5-8F cells and its mechanism. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012; 37(9):871–875. PMID: 23000759.15. Lee K, Kim J, Lee BJ, Park JW, Leem KH, Bu Y. Protective effects of Galla Rhois, the excrescence produced by the sumac aphid, Schlechtendalia chinensis, on transient focal cerebral ischemia in the rat. J Insect Sci. 2012; 12:10.16. Park EJ, Zhao YZ, An RB, Kim YC, Sohn DH. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose from Galla Rhois protects primary rat hepatocytes from necrosis and apoptosis. Planta Med. 2008; 74(11):1380–1383. PMID: 18622905.17. Lee YH. Dyeing, fastness, and deodorizing properties of cotton, silk, and wool fabrics dyed with coffee sludge (Coffea arabica L.) extract. J Appl Polym Sci. 2007; 103(1):251–257.18. Newman DJ. Natural products as leads to potential drugs: an old process or the new hope for drug discovery? J Med Chem. 2008; 51(9):2589–2599. PMID: 18393402.
Article19. Efange SMN. Natural products: a continuing source of inspiration for the medical chemist. In : Iwu MM, Wootton JC, editors. Advances in phytomedicine. Amsterdam: Elsevier Science;2002.20. Cragg GM, Newman DJ. Drug discovery and development from natural products: the way forward. 11th NAPRECA Symposium Book of Proceedings. 2005. p. 56–69.21. Yagi K. Assay for blood plasma or serum. Methods Enzymol. 1984; 105:328–331. PMID: 6727672.22. Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993; 49(3):481–493. PMID: 8221017.
Article23. Jacob RA, Burri BJ. Oxidative damage and defense. Am J Clin Nutr. 1996; 63(6):985–990.
Article24. Mohamed EI, Elazomi A, Elabid BEH, Zwaik H. Evaluation of changes in levels of plasma MDA, and antioxidant vitamin E in Sudanese patients with type 2 diabetes. In : International Conference on Chemical, Environment & Biological Sciences; 2014. p. 17–18.25. Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012; 2012:137289. PMID: 23119185.
Article26. Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ, Morrow JD. Lipid peroxidation in aging brain and Alzheimer's disease. Free Radic Biol Med. 2002; 33(5):620–626. PMID: 12208348.27. Dahanukar SA, Kulkarni RA, Rege NN. Pharmacology of medicinal plants and natural products. Indian J Pharmacol. 2000; 32:S81–S118.28. Dragoev SG. Inhibition of lipid peroxidation of frozen mackerel by pre-storage antioxidant superficial treatment. Bul J Agri Sci. 2008; 14(3):283–289.29. Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sci. 2000; 66(8):709–723. PMID: 10680579.
Article30. Chen CH, Liu TZ, Chen CH, Wong CH, Chen CH, Lu FJ, Chen SC. The efficacy of protective effects of tannic acid, gallic acid, ellagic acid, and propyl gallate against hydrogen peroxide-induced oxidative stress and DNA damages in IMR-90 cells. Mol Nutr Food Res. 2007; 51(8):962–968. PMID: 17628875.
Article31. Nabavi SF, Habtemariam S, Sureda A, Hajizadeh Moghaddam A, Daglia M, Nabavi SM. In vivo protective effects of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress in rat erythrocytes. Arh Hig Rada Toksikol. 2013; 64(4):553–559. PMID: 24384762.32. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999; 15(6):412–426. PMID: 10634967.
Article33. KREBS HA. The physiological role of the ketone bodies. Biochem J. 1961; 80:225–233. PMID: 13754174.
Article34. VanItallie TB, Nufert TH. Ketones: metabolism's ugly duckling. Nutr Rev. 2003; 61(10):327–341. PMID: 14604265.
Article35. Ferrell KE, Thorington RW. Squirrels: the animal answer guide. Johns Hopkins University Press;2006. p. 91.36. Muller-Harvey I, McAllan AB. Tannins: Their biochemistry and nutritional properties. Adv Plant Cell Biochem Biotechnol. 1992; 1:151–217.37. Dollahite JW, Pigeon RF, Camp BJ. The toxicity of gallic acid, pyrogallol, tannic acid, and Quercus havardi in the rabbit. Am J Vet Res. 1962; 23(97):1264–1267. PMID: 14028469.38. Boyd EM, Bereczky K, Godi I. The acute toxicity of tannic acid administered intragastrically. Can Med Assoc J. 1965; 92(25):1292–1297. PMID: 14291458.39. Toxicological Evaluation of some extraction solvents and certain other substances. FAO Nutrition Meetings Series, No. 48A. 1970.40. Inert Reassessmen-t Tannin (CAS Reg. No. 1401-55-4). Office of prevention, pesticides and toxic substances, US Environmental Protection Agency;2006.41. Eaton GJ, Johnson FN, Custer RP, Crane AR. The Icr:Ha(ICR) mouse: a current account of breeding, mutations, diseases and mortality. Lab Anim. 1980; 14(1):17–24. PMID: 7359882.
Article43. Takeno S, Hirano Y, Kitamura A, Sakai T. Comparative developmental toxicity and metabolism of nitrazepam in rats and mice. Toxicol Appl Pharmacol. 1993; 121(2):233–238. PMID: 8346540.
Article44. Xu X, Zhang L, Shao B, Sun X, Ho CT, Li S. Safety evaluation of meso-zeaxanthin. Food Control. 2013; 32(2):678–686.
Article45. Zhang Y, Li J, Wu Z, Liu E, Shi P, Han L, Guo L, Gao X, Wang T. Acute and long-term toxicity of mango leaves extract in mice and rats. Evid Based Complement Alternat Med. 2014; 2014:691574. PMID: 25246938.
Article46. Xiang F, Peng L, Yin Z, Jia R, Hu Z, Li Z, Ni X, Liang X, Li L, He C, Yin L, Su G, Lv C. Acute and subchronic toxicity as well as evaluation of safety pharmacology of Galla chinensis solution. J Ethnopharmacol. 2015; 162:181–190. PMID: 25540924.47. Bissell DM, Gores GJ, Laskin DL, Hoofnagle JH. Drug-induced liver injury: mechanisms and test systems. Hepatology. 2001; 33(4):1009–1013. PMID: 11283870.
Article48. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Nonalcoholic Steatohepatitis clinical research network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41(6):1313–1321. PMID: 15915461.
Article49. Giannini E, Botta F, Fasoli A, Ceppa P, Risso D, Lantieri PB, Celle G, Testa R. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999; 44(6):1249–1253. PMID: 10389705.50. Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998; 93(1):44–48. PMID: 9448172.
Article51. Yoon SH, Park EJ, Oh KH, Chung YG, Kwon OJ. The effect of Lithospermi radix on benzo (a) pyrene-induced hepatotoxicity. J Korean Soc Food Sci Nutr. 1993; 22(2):144–148.52. Hayes AW. Principles and methods of toxicology. New York: Raven Press;1982. p. 447–474.53. Kim DH, Deung YK, Lee YM, Yoon YS, Kwon KR, Park DB, Park YK, Lee KJ. The liver protecting effect of pomegranate (Punica granatum) seed oil in mice treated with CCl4. Korean J Electron Microscopy. 2006; 36(3):173–182.54. Michael B, Yano B, Sellers RS, Perry R, Morton D, Roome N, Johnson JK, Schafer K, Pitsch S. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol Pathol. 2007; 35(5):742–750. PMID: 17849357.
Article55. Singhal PC, Sharma P, Sanwal V, Prasad A, Kapasi A, Ranjan R, Franki N, Reddy K, Gibbons N. Morphine modulates proliferation of kidney fibroblasts. Kidney Int. 198; 53(2):350–357. PMID: 9461094.
Article56. Falconer IR, Hardy SJ, Humpage AR, Froscio SM, Tozer GJ, Hawkins PR. Hepatic and renal toxicity of the blue-green alga (cyanobacterium) Cylindrospermopsis raciborskii in male Swiss albino mice. Environ Toxicol. 1999; 14(1):143–150.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antifungal Activity of the Extracts from Galla rhois against Candida albicans
- Regulation of gastrointestinal hormones during laxative activity of gallotannin-enriched extract isolated from Galla Rhois in loperamide-induced constipation of SD rats
- Identification and partial purification of antibacterial compounds against Streptococcus mutans from Galla Rhois
- Effect of Hexane Extract of Galla Rhois on Inflammatory Alveolar Bone Loss
- Hepatotoxicity and nephrotoxicity of saponin-enriched extract of Asparagus cochinchinensis in ICR mice