Lab Anim Res.
2017 Jun;33(2):57-67. 10.5625/lar.2017.33.2.57.
Hepatotoxicity and nephrotoxicity of saponin-enriched extract of Asparagus cochinchinensis in ICR mice
- Affiliations
-
- 1College of Natural Resources and Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Gangrim Organics, Miryang, Korea.
- 3College of Human Ecology, Pusan National University, Busan, Korea.
- 4Department of Clinical Laboratory Science, College of Nursing and Healthcare Science, Dong-Eui University, Busan, Korea.
- KMID: 2407417
- DOI: http://doi.org/10.5625/lar.2017.33.2.57
Abstract
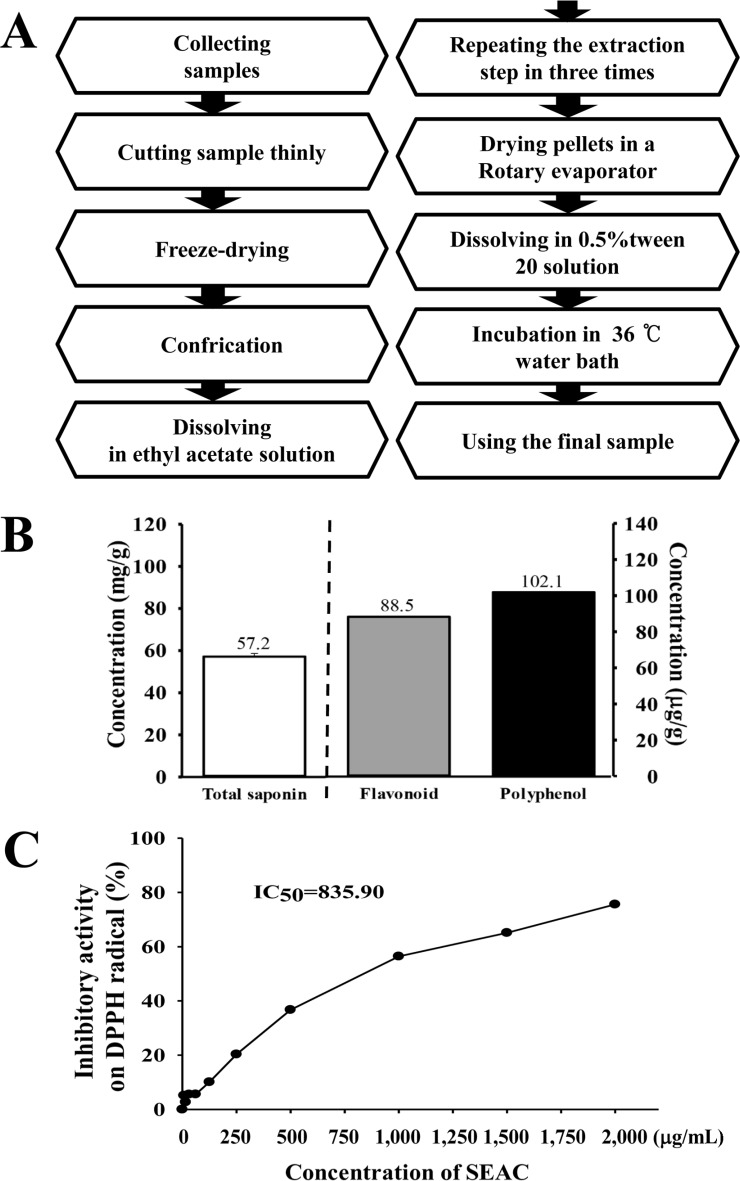
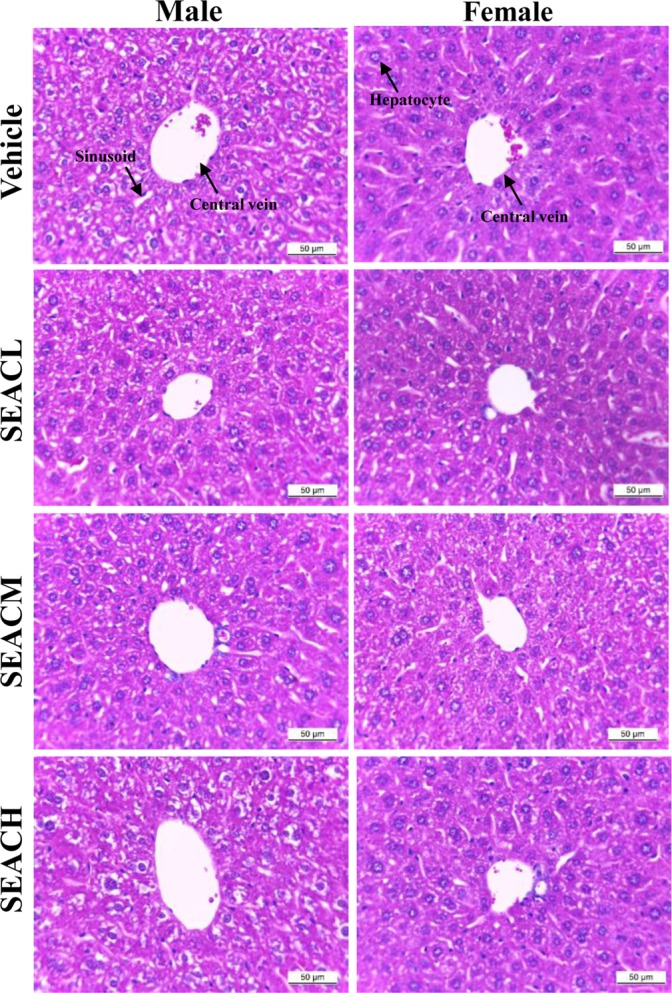
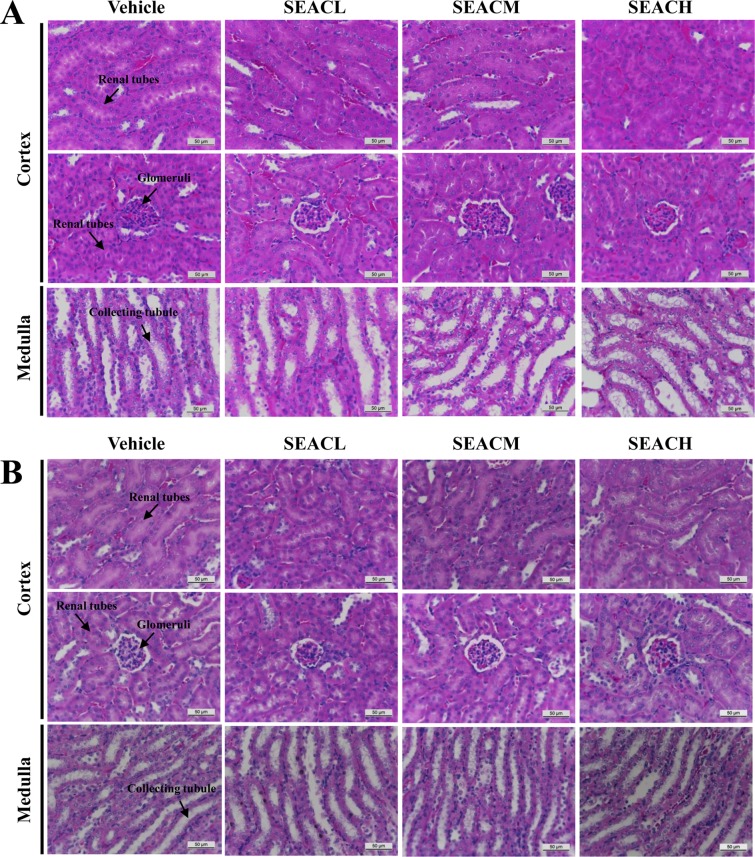
- The inhibitory effects of Asparagus cochinchinensis against inflammatory response induced by lipopolysaccharide (LPS), substance P and phthalic anhydride (PA) treatment were recently reported for some cell lines and animal models. To evaluate the hepatotoxicity and nephrotoxicity of A. cochinchinensis toward the livers and kidneys of ICR mice, alterations in related markers including body weight, organ weight, urine composition, liver pathology and kidney pathology were analyzed in male and female ICR mice after oral administration of 150, 300 and 600 mg/kg body weight/day saponin-enriched extract of A. cochinchinensis (SEAC) for 14 days. The saponin, total flavonoid and total phenol levels were found to be 57.2, 88.5 and 102.1 mg/g in SEAC, respectively, and the scavenging activity of SEAC gradually increased in a dose-dependent manner. Moreover, body and organ weight, clinical phenotypes, urine parameters and mice mortality did not differ between the vehicle and SEAC treated group. Furthermore, no significant alterations were measured in alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), blood urea nitrogen (BUN) and the serum creatinine (Cr) in the SEAC treated group relative to the vehicle treated group. Moreover, the specific pathological features induced by most toxic compounds were not observed upon liver and kidney histological analysis. Overall, the results of the present study suggest that SEAC does not induce any specific toxicity in the livers and kidneys of male and female ICR mice at doses of 600 mg/kg body weight/day.
MeSH Terms
-
Administration, Oral
Alanine Transaminase
Alkaline Phosphatase
Animals
Aspartate Aminotransferases
Blood Urea Nitrogen
Body Weight
Cell Line
Creatinine
Female
Humans
Kidney
L-Lactate Dehydrogenase
Liver
Male
Mice
Mice, Inbred ICR*
Models, Animal
Mortality
Organ Size
Pathology
Phenol
Phenotype
Saponins
Substance P
Alanine Transaminase
Alkaline Phosphatase
Aspartate Aminotransferases
Creatinine
L-Lactate Dehydrogenase
Phenol
Saponins
Substance P
Figure
Reference
-
1. Xiong D, Yu LX, Yan X, Guo C, Xiong Y. Effects of root and stem extracts of Asparagus cochinchinensis on biochemical indicators related to aging in the brain and liver of mice. Am J Chin Med. 2011; 39(4):719–726. PMID: 21721152.2. Xiao PG. Modern chinese material medica. Beijing: Chemical Industry Press;2002. p. 150.3. Kim H, Lee E, Lim T, Jung J, Lyu Y. Inhibitory effect of Asparagus cochinchinensis on tumor necrosis factor-alpha secretion from astrocytes. Int J Immunopharmacol. 1998; 20(4-5):153–162. PMID: 9730251.4. Lee DY, Choo BK, Yoon T, Cheon MS, Lee HW, Lee AY, Kim HK. Anti-inflammatory effects of Asparagus cochinchinensis extract in acute and chronic cutaneous inflammation. J Ethnopharmacol. 2009; 121(1):28–34. PMID: 18691647.5. Luo J, Long QD, Li CX, Li L, Huang NH, Nie M, Tang PX. Comparison of antitussive, expectorant and anti-asthmatic effect between ALWB and ACM. J GuiYang Med Coll. 1998; 23:132–134.6. Sung JE, Lee HA, Kim JE, Go J, Seo EJ, Yun WB, Kim DS, Son HJ, Lee CY, Lee HS, Hwang DY. Therapeutic effect of ethyl acetate extract from Asparagus cochinchinensis on phthalic anhydride-induced skin inflammation. Lab Anim Res. 2016; 32(1):34–45. PMID: 27051441.7. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965; 16(3):144–158.8. Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999; 64:555–559.
Article9. Helaly FM, Soliman HSM, Soheir AD, Ahmed AA. Controlled release of migration of molluscicidal saponin from different types of polymers containing Calendula officinalis. Adv Polym Techn. 2001; 20(4):305–311.10. Oh H, Ko EK, Kim DH, Jang KK, Park SE, Lee HS, Kim YC. Secoiridoid glucosides with free radical scavenging activity from the leaves of Syringa dilatata. Phytother Res. 2003; 17(4):417–419. PMID: 12722154.11. Cragg GM, Newman DJ. Drug discovery and development from natural products: the way forward. In : 11th NAPRECA, Symposium Book of Proceedings; Antananarivo. 2005. p. 56–69.12. Newman DJ. Natural products as leads to potential drugs: an old process or the new hope for drug discovery? J Med Chem. 2008; 51(9):2589–2599. PMID: 18393402.
Article13. Efange SMN. Natural products: a continuing source of inspiration for the medical chemist. In : Wootton JC, editor. Advances in phytomedicine. 5nd ed. Amsterdam: Elsevier Science;2002. p. 61–69.14. Parasuraman S. Toxicological screening. J Pharmacol Pharmacother. 2011; 2(2):74–79. PMID: 21772764.
Article15. Bruneton J. Pharmacognosie, Phytochimie, Plantes Médicinales. Paris: ditions Technique & Documentation;1995. p. 1120.16. Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002; 88(6):587–605. PMID: 12493081.
Article17. Sparg SG, Light ME, van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol. 2004; 94(2-3):219–243. PMID: 15325725.
Article18. Sahu NP, Banerjee S, Mondal NB, Mandal D. Steroidal saponins. Fortschritte der Chemie Organischer Naturstoffe/progress in the chemistry of organic natural products, fortschritte der chemie organischer naturstoffe/progress in the chemistry of organic natural products, vol 89. Vienna: Springer;2008. p. 45–141.19. Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem Rev. 2010; 9(3):425–474. PMID: 20835386.
Article20. Alaoui K, Belabbes M, Cherrah Y, Hassar M, Charrouf Z, Amarouch H, Roquebert J. Acute and chronic toxicity of saponins from Argania spinosa. Ann Pharm Fr. 1998; 56(5):213–219. PMID: 9805821.21. Witthawaskul P, Panthong A, Kanjanapothi D, Taesothikul T, Lertprasertsuke N. Acute and subacute toxicities of the saponin mixture isolated from Schefflera leucantha Viguier. J Ethnopharmacol. 2003; 89(1):115–121. PMID: 14522442.22. Wisløff H, Uhlig S, Scheie E, Loader J, Wilkins A, Flåøyen A. Toxicity testing of saponin-containing Yucca schidigera Roetzl. juice in relation to hepato- and nephrotoxicity of Narthecium ossifragum (L.) Huds. Toxicon. 2008; 51(1):140–150. PMID: 17942132.23. Cho YM, Imai T, Ito Y, Takami S, Hasumura M, Yamazaki T, Hirose M, Nishikawa A. A 13-week subchronic toxicity study of dietary administered saponin-rich and isoflavones-containing soybean extract in F344 rats. Food Chem Toxicol. 2009; 47(8):2150–2156. PMID: 19501625.
Article24. Bissell DM, Gores GJ, Laskin DL, Hoofnagle JH. Drug-induced liver injury: mechanisms and test systems. Hepatology. 2001; 33(4):1009–1013. PMID: 11283870.
Article25. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41(6):1313–1321. PMID: 15915461.
Article26. Giannini E, Botta F, Fasoli A, Ceppa P, Risso D, Lantieri PB, Celle G, Testa R. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999; 44(6):1249–1253. PMID: 10389705.27. Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998; 93(1):44–48. PMID: 9448172.
Article28. Yoon SH, Park EJ, Oh KH, Chung YG, Kwon OJ. The effect of Lithospermi radix on benzo (a) pyrene-induced hepatotoxicity. J Korean Soc Food Sci Nutr. 1993; 22(2):144–148.29. Hayes AW. Principles and methods of toxicology. New York: Raven Press;1982. p. 447–474.30. Kim DH, Deung YK, Lee YM, Yoon YS, Kwon KR, Park DB, Park YK, Lee KJ. The liver protecting effect of pomegranate (Punica granatum) seed oil in mice treated with CCl4. Korean J Electron Microsc. 2006; 36(3):173–182.31. Michael B, Yano B, Sellers RS, Perry R, Morton D, Roome N, Johnson JK, Schafer K, Pitsch S. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol Pathol. 2007; 35(5):742–750. PMID: 17849357.
Article32. Singhal PC, Sharma P, Sanwal V, Prasad A, Kapasi A, Ranjan R, Franki N, Reddy K, Gibbons N. Morphine modulates proliferation of kidney fibroblasts. Kidney Int. 1998; 53(2):350–357. PMID: 9461094.
Article33. Falconer IR, Hardy SJ, Humpage AR, Froscio SM, Tozer GJ, Hawkins PR. Hepatic and renal toxicity of the blue-green alga (cyanobacterium) Cylindrospermopsis raciborskii in male Swiss albino mice. Environ Toxicol. 1999; 14(1):143–150.34. Horiguchi H, Oguma E, Kayama F, Sato M, Fukushima M. Dexamethasone prevents acute cadmium-induced hepatic injury but exacerbates kidney dysfunction in rabbits. Toxicol Appl Pharmacol. 2001; 174(3):225–234. PMID: 11485383.
Article35. Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol. 2010; 28(5):436–440. PMID: 20458311.
Article36. Ezejiofor AN, Orish CN, Orisakwe OE. Effect of aqueous leaves extract of Costus afer Ker Gawl (Zingiberaceae) on the liver and kidney of male albino Wistar rat. Anc Sci Life. 2013; 33(1):4–9. PMID: 25161323.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dose dependence and durability of the therapeutic effects of Asparagus cochinchinensis fermented extract in an ovalbumin-challenged asthma model
- Hepatotoxicity and nephrotoxicity of gallotannin-enriched extract isolated from Galla Rhois in ICR mice
- Therapeutic effect of ethyl acetate extract from Asparagus cochinchinensis on phthalic anhydride-induced skin inflammation
- Four amino acids as serum biomarkers for anti-asthma effects in the ovalbumin-induced asthma mouse model treated with extract of Asparagus cochinchinensis
- The New Phytoformula Containing Morus alba, Schizandra sinensis and Asparagus cochinchinensis Inhibits Lung Inflammation in vitro and in vivo